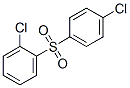
2-Chlorophenyl 4-chlorophenyl sulfone synthesis
- Product Name:2-Chlorophenyl 4-chlorophenyl sulfone
- CAS Number:38980-51-7
- Molecular formula:
- Molecular Weight:0
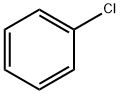
108-90-7
644 suppliers
$10.00/25G
80-07-9
324 suppliers
$6.00/25g
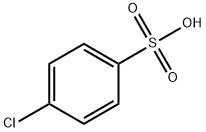
98-66-8
200 suppliers
$5.00/25g

38980-51-7
4 suppliers
inquiry
Yield:98-66-8 67 %Chromat.
Reaction Conditions:
with sulfur trioxide at 75; for 2 h;Inert atmosphere;
Steps:
13 Example 13 : use of MCB in sulfonation reaction
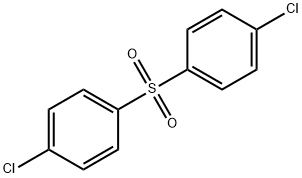
The MCB produced in example 8 was washed with the same volume of 0.02 % aq. potassium hydroxide solution, then washed again with 3 times the same volume of deionized water. The final moisture content of the MCB was approx. 1000 ppm by weight as measured by Karl-Fisher titration. In a dry 3 -neck 250-mL round bottom flask, containing a PTFE-coated stir bar and fitted with a thermocouple, a distillation receiver allowing returning the vapors to the mixture (Barrett trap) + reflux condenser + H2SO4 scrubber, and a inlet tube connected to an oleum distillation set up and a nitrogen inlet, was introduced under nitrogen 90.06 g of MCB from example 8 neutralized as detailed above (0.80 mol). The flask was then sealed and the mixture was heated to 75°C under agitation. When the mixture had reached 75°C, 55.00 g of SO3 vapors (0.14 mol) were slowly introduced to the reactor from an adjacent oleum distillation set up. The addition lasted 60 minutes, during which the temperature was maintained at 75°C by applying external cooling to the reaction flask. At the end of the addition, the reaction mixture was held at 75°C for 1 hour. At the end of the reaction, the mixture was analyzed by GC and found to contain : ? 61 wt % chlorobenzenesulfonic acid (0.49 mol, 67 % yield, 95.7 % pCBSA/4.3 % oCBSA) ? 14 wt % DCDPS (0.07 mol, 9 % yield, 90 % / 5.5 % / 3.5 % 4,472,473,4') ? 9 wt H2S04 (0.13 mol, 19 % yield) ? 16 wt % MCB (0.19 mol).
References:
WO2013/87594,2013,A1 Location in patent:Page/Page column 18; 19

98-66-8
200 suppliers
$5.00/25g

108-90-7
644 suppliers
$10.00/25G

80-07-9
324 suppliers
$6.00/25g

38980-51-7
4 suppliers
inquiry

98-66-8
200 suppliers
$5.00/25g

108-90-7
644 suppliers
$10.00/25G

80-07-9
324 suppliers
$6.00/25g

38980-51-7
4 suppliers
inquiry
![Benzene, 1-chloro-3-[(4-chlorophenyl)sulfonyl]-](/CAS/20210111/GIF/38980-69-7.gif)
38980-69-7
3 suppliers
inquiry
98-60-2
323 suppliers
$5.00/5g

108-90-7
644 suppliers
$10.00/25G

80-07-9
324 suppliers
$6.00/25g

38980-51-7
4 suppliers
inquiry