
2-CHLOROQUINOLINE-3-BORONIC ACID synthesis
- Product Name:2-CHLOROQUINOLINE-3-BORONIC ACID
- CAS Number:128676-84-6
- Molecular formula:C9H7BClNO2
- Molecular Weight:207.42
612-62-4
250 suppliers
$35.00/5g

5419-55-6
370 suppliers
$12.00/5g

7732-18-5
463 suppliers
$13.50/100ML

128676-84-6
118 suppliers
$45.00/250mg
Yield:128676-84-6 77%
Reaction Conditions:
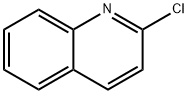
Stage #1: 2-Chloroquinolinewith lithium diisopropyl amide in tetrahydrofuran at -40; for 1.5 h;Inert atmosphere;
Stage #2: Triisopropyl borate in tetrahydrofuran; for 2 h;Inert atmosphere;
Stage #3: waterwith hydrogenchlorideInert atmosphere;Solvent;Reagent/catalyst;Temperature;
Steps:
1-6; 10 Example 1:
Under nitrogen, 400ml of tetrahydrofuran and 2-chloroquinoline (40g; 0.25mol) were sequentially added to a 1000ml three-necked flask. Turn on the stirring, lower the temperature to -40°C, react for 0.5 hours, then add lithium diisopropylamide (150ml, 0.38mol) dropwise, keep the temperature at -40°C and react for one hour and start adding triisopropyl borate (71g, 0.38). mol), the reaction is kept for two hours. Post-treatment: 400ml 1mol/L dilute hydrochloric acid was added to the reaction to quench the reaction, the system was extracted with 400ml ethyl acetate, the organic phase was separated, and the organic phase was dried (drying with anhydrous magnesium sulfate at room temperature for 1 to 3 hours) Concentrate under reduced pressure (at a temperature of 30-50° C., pumped to a negative pressure with a vacuum water pump to remove tetrahydrofuran and ethyl acetate in the system), and recrystallized with petroleum ether to obtain 39.1 g of white solid. HPLC=99%, the yield was 77%.
References:
CN113004312,2021,A Location in patent:Paragraph 0046-0063; 0073-0075

612-62-4
250 suppliers
$35.00/5g
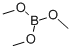
121-43-7
345 suppliers
$14.00/25mL

7732-18-5
463 suppliers
$13.50/100ML

128676-84-6
118 suppliers
$45.00/250mg

612-62-4
250 suppliers
$35.00/5g

688-74-4
321 suppliers
$10.40/25mL

7732-18-5
463 suppliers
$13.50/100ML

128676-84-6
118 suppliers
$45.00/250mg