
2-CYANO-4-PHENYLPYRIDINE synthesis
- Product Name:2-CYANO-4-PHENYLPYRIDINE
- CAS Number:18714-16-4
- Molecular formula:C12H8N2
- Molecular Weight:180.21
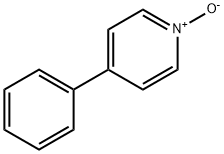
1131-61-9
102 suppliers
$39.00/1g
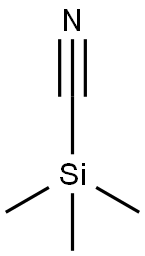
7677-24-9
373 suppliers
$19.00/5g

18714-16-4
24 suppliers
$236.81/1gm:
Yield:18714-16-4 89%
Reaction Conditions:
with N,N-Dimethylcarbamoyl chloride in Nitroethane at 20; for 45 h;
Steps:
34 Reference Example 34
Reference Example 34 2-Cyano-4-phenylpyridine 4-Phenylpyridine N-oxide (3.13 g, 18.3 mmol) was dissolved in nitroethane (30 ml), and trimethylsilyl cyanide (2.0 g, 20.2 mmol) and N,N-dimethylcarbamoyl chloride (1.7 ml, 18.5 mmol) were added thereto.. The reaction mixture was stirred at room temperature for 45 hrs, concentrated under reduced pressure, combined with saturated aqueous sodium hydrogen carbonate solution and extracted with ethyl acetate.. The extract was washed with saturated brine and dried.. The solvent was evaporated under reduced pressure.. The obtained crystals were collected by filtration, washed with diisopropyl ether and dried to give the titled compound (2.87 g, 89 %).1H-NMR (CDCl3) δ: 7.47-7.65 (5H, m), 7.72 (1H, dd, J = 1.8, 5.2 Hz), 7.91 (1H, s), 8.75 (1H, d, J = 5.1 Hz).
References:
EP1424336,2004,A1 Location in patent:Page 104
19235-89-3
264 suppliers
$10.00/1g

98-80-6
714 suppliers
$5.00/5g

18714-16-4
24 suppliers
$236.81/1gm:
62150-45-2
195 suppliers
$14.00/1g

98-80-6
714 suppliers
$5.00/5g

18714-16-4
24 suppliers
$236.81/1gm:

62150-45-2
195 suppliers
$14.00/1g
591-51-5
127 suppliers
$123.00/50ml

18714-16-4
24 suppliers
$236.81/1gm:

19235-89-3
264 suppliers
$10.00/1g

24388-23-6
205 suppliers
$6.00/5g

18714-16-4
24 suppliers
$236.81/1gm: