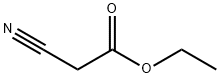
2-cyano-N-[2-(1H-indol-3-yl)ethyl]acetamide synthesis
- Product Name:2-cyano-N-[2-(1H-indol-3-yl)ethyl]acetamide
- CAS Number:103344-22-5
- Molecular formula:C13H13N3O
- Molecular Weight:227.26
Yield:103344-22-5 79%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in dichloromethane at 20;
Steps:
2.1 Step 1: Preparation of N-[2-(1H-indol-3-yl) ethyl]-2-cyano acetamide
Step 1:
Preparation of N-[2-(1H-indol-3-yl) ethyl]-2-cyano acetamide
2-(1H-indol-3-yl) ethylamine (16.0 g, 0.1 mol), 2-cyanoacetate acid (8.5 g, 0.1 mol), EDCI (19.2 g, 0.1 mol), HOBt (13.5 g, 0.1 mol) and Et3N (20.2 g, 0.2 mol) were dissolved in CH2Cl2 (200 mL), and stirred at room temperature overnight.
Distilled water (100 mL) was added, the liquid layers obtained was separated, the aqueous layer was extracted with CH2Cl2 (80 mL*2), the mixed organic layer was successively washed with distilled water (80 mL*2) and saturated brine (80 mL*1), dried with Na2SO4, concentrated.
The residue was separated by a silica gel column (PE/EA=1:1) to give 18.0 g of a white solid with a yield of 79.0%.
MS Detection:
MASS(ESI+) m/z=228 (M+H)+.
References:
US2016/272604,2016,A1 Location in patent:Paragraph 0060; 0061; 0062; 0063