2-CYANO-N-(4-ETHOXY-PHENYL)-ACETAMIDE synthesis
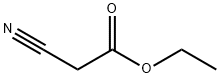
- Product Name:2-CYANO-N-(4-ETHOXY-PHENYL)-ACETAMIDE
- CAS Number:51838-07-4
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23
Yield:51838-07-4 78%
Reaction Conditions:
with acetic anhydride at 85; for 0.5 h;
Steps:
Synthesis of 2-cyano-N-(4-ethoxyphenyl)acetamide (2)
To a warm solution of cyanoacetic (50 mmol) and acetic anhydride (50 mmol) at 50C, wasadded (50 mmol) of 4-ethoxyaniline. The mixture was heated to 85C for 25 min, whereupon the product started to crystallize. After a further 5 min, the mixture was allowedto cool to room temperature, and the resulting solid product was collected by filtration,washed with methanol, dried in air and recrystallized from dioxane. Grey crystals, yield(78%), mp 178-180C; IR (KBr) νmax/cm-1: 3297 (NH), 3096 (CH-Ar), 2973 (CH- sp3),2254 (CN), 1662 (CO); 1H-NMR (DMSO-d6): δppm = 1.31 (t, J = 7 Hz, 3H, CH3), 3.85 (s,2H, COCH2), 3.98 (q, J = 7 Hz, 2H, CH2), 6.89 (d, J = 9 Hz, 2H, CHAr), 7.45 (d, J = 9Hz, 2H, CHAr), 10.13 (s, 1H, NH); 13C-NMR (DMSO-d6): δppm = 14.6, 26.4, 63.1, 114.5(2C), 115.9, 120.8 (2C), 131.3, 154.8, 160.3; MS m/z (%): 204 (M+, 86), 176 (45), 135(29), 108 (100), 80 (7), 68 (11); Anal. Calcd. for C11H12N2O2 (204): C, 64.69; H, 5.92; N,13.72%, Found: C, 64.65; H, 5.95; N, 13.76%.
References:
Bondock, Samir;Nasr, Tamer;Zaghary, Wafaa;Chantrapromma, Suchada;Ghabbour, Hazem;Fun, Hoong-Kun [Molecular Crystals and Liquid Crystals,2014,vol. 605,# 1,p. 165 - 178]
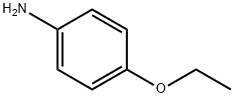
156-43-4
248 suppliers
$5.00/5G
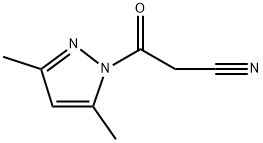
36140-83-7
87 suppliers
$23.00/250mg

51838-07-4
11 suppliers
inquiry