
2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide synthesis
- Product Name:2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide
- CAS Number:24522-30-3
- Molecular formula:C10H7F3N2O
- Molecular Weight:228.17
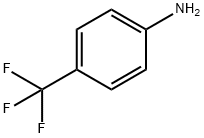
455-14-1
503 suppliers
$5.00/10g

372-09-8
350 suppliers
$20.00/25g
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
125 suppliers
$21.00/250mg
Yield: 95%
Reaction Conditions:
Stage #1:cyanoacetic acid with 4-methyl-morpholine in tetrahydrofuran at 0 - 10; for 1 h;Industry scale;
Stage #2:4-trifluoromethylphenylamine with isopropyl chloroformate in tetrahydrofuran at 0 - 10; for 2 - 3 h;Product distribution / selectivity;Industry scale;
Steps:
1; 1 Preparation of 4-trifluoromethyl cyanoacetoanilide (compound (IV))
Example 1 Preparation of 4-trifluoromethyl cyanoacetoanilide (compound (IV)) In 700 L of tetrahydrofuran, 63.4 kg of cyanoacetic acid was dissolved with stirring in nitrogen atmosphere at room temperature. This solution was cooled to 0 to 10°C, and then N-methylmorpholine was added dropwise with stirring at the same temperature in about an hour. Then, 100.0 kg of 4-trifluoromethylaniline was added dropwise. To this reaction mixture, 91.3kg of isopropyl chlorocarbonate was added dropwise with stirring at the same temperature in about an hour. Further, the stirring was continued for 1 to 2 hours. After the reaction was finished, 200 L of water was added to the reaction mixture, which was stirred and then allowed to stand for separation. An organic layer (upper layer) was washed with 16.7 % brine, and then 400 L of isopropyl alcohol was added thereto, which was concentrated under reduced pressure until the liquid amount became 400 L. To the condensed solution, 400 L of isopropyl alcohol was added, which was concentrated again under reduced pressure until the liquid amount became 400 L. To the condensed solution, 100 L of isopropyl alcohol was added at 20 to 30°C with stirring, and then 500 L of water was added dropwise in about an hour. Then, the stirring was further continued for an hour at the same temperature. This mixture was cooled to 0 to 10°C with stirring, and then stirred at the same temperature for an hour. Precipitated crystals were collected by filtration and dried, to give 134.5 kg of the title compound (IV) in a yield of 95.0 %.
References:
Astellas Pharma Inc. EP1609778, 2005, A1 Location in patent:Page/Page column 5; 8-9

455-14-1
503 suppliers
$5.00/10g

16130-58-8
18 suppliers
inquiry
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
125 suppliers
$21.00/250mg

455-14-1
503 suppliers
$5.00/10g
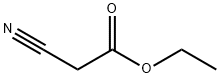
105-56-6
433 suppliers
$10.00/5ml
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
125 suppliers
$21.00/250mg

455-14-1
503 suppliers
$5.00/10g
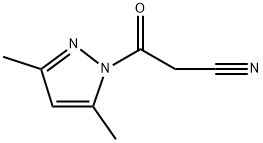
36140-83-7
83 suppliers
$23.00/250mg
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
125 suppliers
$21.00/250mg