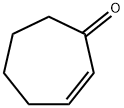
2-Cyclohepten-1-one synthesis
- Product Name:2-Cyclohepten-1-one
- CAS Number:1121-66-0
- Molecular formula:C7H10O
- Molecular Weight:110.15
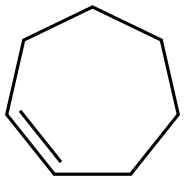
628-92-2
73 suppliers
$60.00/500mg

1121-66-0
120 suppliers
$11.00/1g
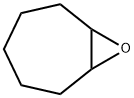
286-45-3
24 suppliers
$45.00/100mg
Yield:-
Reaction Conditions:
with oxygen in acetonitrile at 120; under 3800.26 Torr; for 13 h;
Steps:
2.3.1. Catalytic experiments
Briefly, the required amount of UiO-66(Zr Ti)-X (X: NH2, H and NO2) employed as catalyst (0.016mmol of total metal Zr+Ti) was introduced into a reactor vessel (5mL). Subsequently, the olefin reagent (2mmol) dissolved in CH3CN (2.5mL) was added to the vessel. The system was pressurized with O2 at the required value at room temperature (i.e. 5 or 2atm). The reactions were carried out under 600rpm magnetic stirring to ensure that the process is under kinetic control. Catalyst reusability was studied for the most active sample (UiO-66(Zr5.4Ti0.6)-NO2). At the end of the reaction, the solid catalyst was recovered by filtration (Nylon membrane, 0.2μm) and, transferred to a round-bottom flask (50mL) and washed under magnetic stirring with ethanol (20mL) at 80°C for 2h. This procedure was repeated three times. The washed, used solid catalyst was recovered by filtration (Nylon membrane, 0.2μm) and dried in an oven at 100°C for 24h. Before the new catalytic cycle, the solid catalyst was activated at 150°C under vacuum for 16h. Selective radical quenching experiments were carried out following the general reaction procedure described above, but with the addition of radical quenchers (20mol% with respect to the substrate). In particular, dimethylsulfoxide (DMSO) [53-56] or p-benzoquinone [53,54,56,57] were added as selective hydroxyl or superoxide/hydroperoxyl radical scavengers, respectively.
References:
Santiago-Portillo, Andrea;Navalón, Sergio;Álvaro, Mercedes;García, Hermenegildo [Journal of Catalysis,2018,vol. 365,p. 450 - 463]

502-42-1
209 suppliers
$10.00/1g

1121-66-0
120 suppliers
$11.00/1g
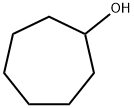
502-41-0
163 suppliers
$53.00/25mL

1121-66-0
120 suppliers
$11.00/1g
![1,4-Dioxaspiro[4.6]undec-6-ene](/CAS/GIF/1728-24-1.gif)
1728-24-1
9 suppliers
inquiry

1121-66-0
120 suppliers
$11.00/1g
![Cycloheptene, 1-[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/22081-48-7.gif)
22081-48-7
0 suppliers
inquiry

1121-66-0
120 suppliers
$11.00/1g