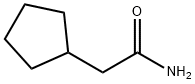
2-CYCLOPENTYLACETAMIDE synthesis
- Product Name:2-CYCLOPENTYLACETAMIDE
- CAS Number:933-04-0
- Molecular formula:C7H13NO
- Molecular Weight:127.18
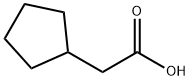
1123-00-8
170 suppliers
$12.00/1g

933-04-0
14 suppliers
inquiry
Yield: 98%
Reaction Conditions:
Stage #1:2-cyclopentylacetic acid with Carbonyldiimidazole in dichloromethane at 22; for 1 h;Inert atmosphere;
Stage #2: with ammonium hydroxide in dichloromethane;water at 0 - 20;
Steps:
Preparation of 2-cyclopentylacetamide (S1)
A flame-dried flask with stir bar was charged with cyclopentylacetic acid (2.56 g, 20.0 mmol, 1.0 equiv) and fitted with a rubber septum. The flask was evacuated and backfilled with N2. ACS grade CH2Cl2 (66 mL, 0.3 M) was added via syringe. Carbonyl diimidazole (CDI, 4.06 g, 25.0 mmol, 1.25 equiv) was then added by briefly removing the septum and adding the solid as asingle portion. The reaction was left to stir at 22 °C for 1 h. After 1 h, the reaction flask wascooled at 0 °C in an ice water bath and aqueous NH4OH (30%) (9.4 mL, 72.4 mmol, 3.6 equiv)) was added. Following NH4OH addition, the reaction was allowed to slowly warm to room temperature by not removing the ice bath and stirred overnight. After stirring overnight, the biphasic suspension was transferred to a separatory funnel anddiluted with saturated aqueous NaHCO3 (ca. amount of CH2Cl2). The reaction flask was rinsed with an additional 5 mL CH2Cl2 to ensure quantitative transfer. The organic phase was removedand the aqueous was extracted once more with CH2Cl2. The combined organic phases were washed with 1 M HCl, dried over Na2SO4, filtered, and concentrated under reduced pressure. Typically, the crude material did not require further chromatographic purification. The product was obtained as a white solid (2.49 g, 98% yield) without chromatographic purification. 1H NMR (400 MHz, CDCl3) δ 5.85 (br s, 1H), 5.53 (br s, 1H), 2.24-2.15 (m, 3H), 1.87-1.82 (m,2H), 1.64-1.50 (m, 4H), 1.19-1.11 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 175.7, 42.1, 36.9, 32.5, 24.9. IR (neat) ν 3346 (br m), 3168 (br m), 2951 (m), 2867 (m), 1661 (s), 1629 (s), 1412 (s), 1353 (m), 1308 (m), 1250 (m), 1156 (m), 952 (w), 882 (w), 809 (w), 672 (s) cm-1. TLC Rf = 0.19 in 1:1 CH2Cl2:EtOAc. HRMS (ESI) m/z: [M + H]+ Calcd for C7H13NO 128.1070; Found 128.1068.
References:
Shehata, Mina F.;Short, Melanie A.;Sanders, Matthew A.;Roizen, Jennifer L. [Tetrahedron,2019,vol. 75,# 24,p. 3186 - 3194] Location in patent:supporting information
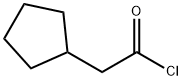
1122-99-2
38 suppliers
$15.88/Price $/250mgs:

933-04-0
14 suppliers
inquiry

5732-87-6
36 suppliers
inquiry

933-04-0
14 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

933-04-0
14 suppliers
inquiry