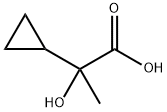
2-Cyclopropyl-2-hydroxy-propionic acid synthesis
- Product Name:2-Cyclopropyl-2-hydroxy-propionic acid
- CAS Number:99848-37-0
- Molecular formula:C6H10O3
- Molecular Weight:130.14
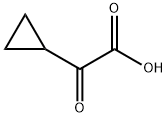
13885-13-7
76 suppliers
$106.00/100mg
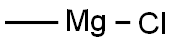
676-58-4
277 suppliers
$15.00/25ml

99848-37-0
16 suppliers
inquiry
Yield:99848-37-0 52 mg
Reaction Conditions:
in tetrahydrofuran at 0 - 20;
Steps:
2 Preparation of compound R3:
add 3a (50mg, 0.44mmol) to a 50mL single-mouth bottle, 3mL of tetrahydrofuran and stir under an ice bath, and add methylmagnesiumbromidedropwise (1M in THF) after the temperature reaches 0 °C; 1.1mL, 1.10mmol), the solution was stirred overnight at room temperature, and then the reaction solution was cooled to 0 °C and quenched with 1N HC1 (10mL), the reaction solution was stirred at room temperature for 2 hours, and thenethylacetate(2*15mL) was used for two extractions, the organic layer was washed with saturated saline, the organic layer was dried with anhydrous magnesium sulfate, and then the organic layer was concentrated to obtain the product R3 (52mg)
References:
CN115850291,2023,A Location in patent:Paragraph 0068-0070
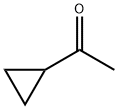
765-43-5
401 suppliers
$10.00/1g

99848-37-0
16 suppliers
inquiry
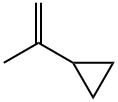
4663-22-3
7 suppliers
inquiry

99848-37-0
16 suppliers
inquiry