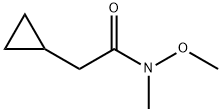
2-Cyclopropyl-N-methoxy-N-methyl-acetamide synthesis
- Product Name:2-Cyclopropyl-N-methoxy-N-methyl-acetamide
- CAS Number:227322-00-1
- Molecular formula:C7H13NO2
- Molecular Weight:143.18
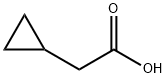
5239-82-7
237 suppliers
$11.00/1g

6638-79-5
576 suppliers
$6.00/25g

227322-00-1
36 suppliers
inquiry
Yield:227322-00-1 98.2%
Reaction Conditions:
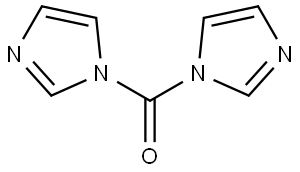
Stage #1: cyclopropylacetic acidwith 1,1'-carbonyldiimidazole in dichloromethane at 22 - 25; for 1.25 h;Large scale;
Stage #2: N,O-dimethylhydroxylamine*hydrochloridewith triethylamine in dichloromethane at 22 - 30; for 1 h;Large scale;
Steps:
15C.1 Step 1 : Preparation of 2-cycl opropyl -L -m ethoxy-A-m ethyl acetam i de (Compound 2-B)
A suspension of 1,1’ -Carbonyl diimidazole (152.6 kg, 1.01 eq.) in DCM (682 kg, 513 L, 7.3 w/w relative to 2-cyclopropylacetic acid) was treated with a solution of 2- cyclopropyl acetic acid (1-B, 93.6 kg, 1 eq.) in DCM (248 kg, 186 L, 2.65 w/w) over at least 1 h, keeping the temperature < 25 °C and compensating for significant effervescence. The resulting mixture is stirred for 15 min at 22 °C and then Af,G-di methyl hydroxylamine*HCl (93.3 kg, 1.03 eq.) is added in portions, keeping the temperature <30 °C. Subsequently, triethylamine (46.4 kg, 0.49 eq.) is added to the stirring mixture at 20 - 25 °C. The resulting mixture is stirred at 22 °C at least 1 h. The mixture is washed once with KHSO4 solution (0.24 M, 357.1 kg, 0.09 eq.), once with KHSO4 solution (0.40 M, 365.4 kg, 0.15 eq.), once with KHSO4 solution (0.80 M, 384.5 kg, 0.30 eq.), and once with NaHCCb solution (0.60 M, 393.1 kg, 0.24 eq.). Residual DCM is removed by three put-and-takes of THF (166.6 kg, 1.78 w/w) and vacuum distillation (50 - 60 °C, to minimum volume/until distillation stops). THF (333.2 kg. 3.56 w/w) is added and the yield is determined by correcting for the LOD and GC- FID purity of the sample (131.5 kg, 98.2% corrected). 1H-NMR (400 MHz, DMSO-i/6) d: - 0.01 - 0.03 (m, 2H) 0.32 - 0.36 (m, 2H) 0.81 - 0.90 (br m, 1H) 2.18 (d, J=6.80 Hz, 2H) 2.97 (s, 3 H) 3.53 (s, 3H). ESI-MS: 144.0 [M+H]+.
References:
WO2021/252669,2021,A1 Location in patent:Page/Page column 156-157

5239-82-7
237 suppliers
$11.00/1g

6638-79-5
576 suppliers
$6.00/25g
530-62-1
941 suppliers
$6.00/25g

227322-00-1
36 suppliers
inquiry

5239-82-7
237 suppliers
$11.00/1g

227322-00-1
36 suppliers
inquiry
54322-65-5
40 suppliers
$45.00/10mg

6638-79-5
576 suppliers
$6.00/25g

227322-00-1
36 suppliers
inquiry
