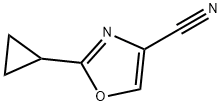
2-cyclopropyloxazole-4-carbonitrile synthesis
- Product Name:2-cyclopropyloxazole-4-carbonitrile
- CAS Number:1159734-36-7
- Molecular formula:C7H6N2O
- Molecular Weight:134.14
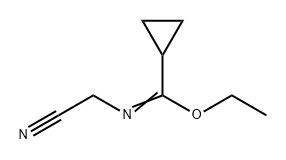
1159734-34-5
0 suppliers
inquiry
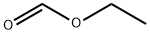
109-94-4
462 suppliers
$10.00/10g

1159734-36-7
12 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-cyclopropyl-4-ethoxy-3-azabut-3-enenitrile;formic acid ethyl esterwith potassium tert-butylate in tetrahydrofuran at -10;
Stage #2: with acetic acid for 0.0333333 h;Heating / reflux;
Steps:
To a solution of N-cyanomethyl-cyclopropanecarboximidic acid ethyl ester (4.54 g, 14.9 mmol) in THF (10 mL) at -10 °C were added potassium t?rt-butoxide (1.67 g, 14.9 mmol) and ethyl formate (1.2 mL, 14.9 mmol) successively. After being stirred at -10 °C for 3 h, the reaction mixture was left in the refrigerator overnight and then diluted with ether. The precipitated brown solid was filtered and dried under vacuum. The vacuum-dried solid was added to boiling acetic acid (45 mL) and refluxed for 2 min. The reaction mixture was cooled to room temperature,
References:
WO2009/70485,2009,A1 Location in patent:Page/Page column 136-137