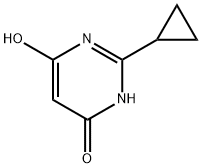
2-Cyclopropylpyrimidine-4,6-diol synthesis
- Product Name:2-Cyclopropylpyrimidine-4,6-diol
- CAS Number:7024-58-0
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15
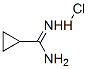
57297-29-7
202 suppliers
$8.00/1g
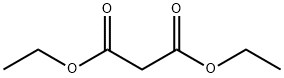
105-53-3
733 suppliers
$5.00/25g

7024-58-0
21 suppliers
$45.00/10mg
Yield:7024-58-0 96%
Reaction Conditions:
Stage #1: cyclopropylcarboxamidine hydrochloridewith methanol;sodium for 0.25 h;Cooling with ice;
Stage #2: diethyl malonate in methanol at 20;Cooling with ice;
Stage #3: with hydrogenchloride in water; pH=5;
Steps:
1
2-Cyclopropylpyrimidine-4,6-diol (2) Sodium metal (5.83 g, 254 mmol) was added to anhydrous methanol (100 mL) cooled in an ice bath. Once sodium methoxide formation was complete (all sodium dissolved), cyclopropyl carboxamidine hydrochloride (10.0 g, 80 mmol) was added and reaction mixture was stirred for 15 min. Diethyl malonate (12.6 imL, 82.9 mmol) was added dropwise and the reaction mixture was warmed to RT and stirred overnight. The suspension was then concentrated under vacuum. The resulting residue was dissolved in water and the solution was acidified with concentrated HCI to pH 5. The resulting precipitate was collected via filtration and washed sequentially with water, ether, isopropanol, and finally ether. The solid was then dried under vacuum to afford 11 .69 g (96 %) of 2-cyclopropylpyrimidine-4,6- diol (2). LCMS m/z 153.4 (M+1 ). H NMR (400 MHz, DMSO-d6) δ 1 1 .87 (s, 2H), 5.03 (s, 1 H), 1.86 (m, 1 H), 0.98 (m, 4H).
References:
WO2011/58478,2011,A1 Location in patent:Page/Page column 30-31

57297-29-7
202 suppliers
$8.00/1g
108-59-8
580 suppliers
$5.00/25g

7024-58-0
21 suppliers
$45.00/10mg
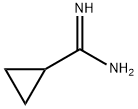
54070-74-5
60 suppliers
$60.00/1g

105-53-3
733 suppliers
$5.00/25g

7024-58-0
21 suppliers
$45.00/10mg
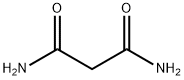
108-13-4
261 suppliers
$9.00/1g
4606-07-9
134 suppliers
$10.00/1g

7024-58-0
21 suppliers
$45.00/10mg