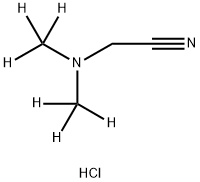
2-[Di(methyl-d3)amino]acetonitrile Hydrochloride synthesis
- Product Name:2-[Di(methyl-d3)amino]acetonitrile Hydrochloride
- CAS Number:1562066-93-6
- Molecular formula:C4H9ClN2
- Molecular Weight:120.58
53170-19-7
86 suppliers
$65.00/500mg

590-17-0
326 suppliers
$5.00/5g
![2-[Di(methyl-d3)amino]acetonitrile Hydrochloride](/CAS/20211123/GIF/1562066-93-6.gif)
1562066-93-6
2 suppliers
inquiry
Yield:1562066-93-6 82%
Reaction Conditions:
Stage #1: d6-dimethylamine hydrochloride;cyanomethyl bromidewith potassium carbonate in tetrahydrofuran at -10 - 20;
Stage #2: with hydrogenchloride in 1,4-dioxane;dichloromethane; for 0.166667 h;
Steps:
A.b
A suspension of deuterated-d6-dimethylamine hydrochloride (18.4 g, 210 mmol) and 2- bromoacetonitrile (14.63 ml, 210 mmol) in anhydrous THF (250 mL) in a round bottom flask was cooled to -10°C with vigorous stirring and treated portion-wise with potassium carbonate (58.1 g, 420 mmol). After addition of the base, the reaction was fitted with a reflux condensor and balloons and allowed to warm slowly to 5°C over 2 hours. TLC (1:1 EtOAc/lso-Hexanes) indicated the presence of product. The mixture was stirred at room temperature over a weekend. The residue was diluted with DCM (250 mL) and filtered, washing with copious amounts of DCM. The mother liquors were degassed with N2 for 1 hour, then reduced in volume by half on the rotavap. Then a 4M dioxane solution of hydrogen chloride (52.5 ml, 210 mmol) was added, precipitating a white solid and the mixture allowed to stand for 10 minutes before being filtered, washing with DCM to afford deuterated-d6- dimethylacetonitrile (21.73 g, 172 mmol, 82 % yield). H NMR (400MHz, d6-DMSO) δ: 4.47 (2H, s) was consistent with the desired material.
References:
WO2014/23956,2014,A1 Location in patent:Page/Page column 32; 33