
2-ETHOXY-4-FLUOROBENZALDEHYDE synthesis
- Product Name:2-ETHOXY-4-FLUOROBENZALDEHYDE
- CAS Number:883537-24-4
- Molecular formula:C9H9FO2
- Molecular Weight:168.16

74-96-4
431 suppliers
$9.00/5g
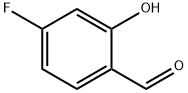
348-28-7
193 suppliers
$21.21/250mgs:

883537-24-4
25 suppliers
$110.00/50mg
Yield:883537-24-4 93%
Reaction Conditions:
with potassium carbonate in acetone at 40; for 16 h;Inert atmosphere;
Steps:
4-fluoro-2-ethoxybenzaldehyde
A flame-dried 100-mL round-bottomed flask equipped with a stirbar under an argon atmosphere was charged with 4-fluoro-2-hydroxy-benzaldehye (1.20 g, 5.97 mmol) in acetone (27 mL), followed by the addition of anhydrous potassium carbonate (1.24 g, 8.95 mmol), and bromoethane (3.25 g, 29.85 mmol, 2.23 mL).
The reaction mixture was fitted with a reflux glycol condenser and heated at 40° C. for 16 hours, and then cooled to room temperature.
The reaction was filtered and the filtered solid was washed with acetone.
The filtrate was evaporated via rotary evaporation to yield 4-fluoro-2-ethoxybenzaldehyde as a yellow-orange solid (1.37 g, 93% yield) that was used without further purification. 1H NMR (CDCl3, 400 MHz) 8 10.41 (s, 1H), 7.85 (m, 1H), 6.73-6.62 (overlap, 2H), 4.13 (q, J=8.0 Hz, 2H), 1.49 (t, J=8.0 Hz, 3H).
References:
US2022/62272,2022,A1 Location in patent:Paragraph 0288; 0289

348-28-7
193 suppliers
$21.21/250mgs:

75-03-6
359 suppliers
$11.00/5g

883537-24-4
25 suppliers
$110.00/50mg

74-96-4
431 suppliers
$9.00/5g

883537-24-4
25 suppliers
$110.00/50mg