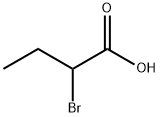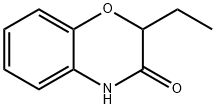
2-Ethyl-2H-benzo[b][1,4]oxazin-3(4H)-one synthesis
- Product Name:2-Ethyl-2H-benzo[b][1,4]oxazin-3(4H)-one
- CAS Number:90921-75-8
- Molecular formula:C10H11NO2
- Molecular Weight:177.2
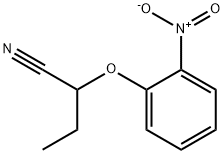
1249621-96-2
0 suppliers
inquiry
![2-Ethyl-2H-benzo[b][1,4]oxazin-3(4H)-one](/CAS/GIF/90921-75-8.gif)
90921-75-8
28 suppliers
$55.00/250mg
Yield:90921-75-8 81%
Reaction Conditions:
with iron;acetic acid for 2.5 h;Inert atmosphere;Reflux;
Steps:
4.2. General procedure for the reductive cyclization (3a-u)
General procedure: To a stirred solution of 2a (2 mmol) in acetic acid (10 mL), powdered Fe (12 mmol) was added and the reaction mixture was then refluxed for 2 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature and the acetic acid was removed under reduced pressure, EtOAc (20 mL) was added and was stirred for 2 min and then filtered to remove any iron impurities. The insoluble iron residue was washed with EtOAc (20 mL). The filtrate and washings were combined and dried over anhydrous MgSO4. The solvent was removed under reduced pressure and the crude product thus obtained was purified by column chromatography to obtain the pure product 3a.
References:
Ramesh, Chintakunta;Raju, B. Rama;Kavala, Veerababurao;Kuo, Chun-Wei;Yao, Ching-Fa [Tetrahedron,2011,vol. 67,# 6,p. 1187 - 1192] Location in patent:experimental part
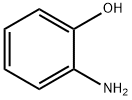
95-55-6
539 suppliers
$5.00/5G
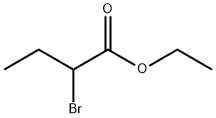
533-68-6
375 suppliers
$5.00/10g
![2-Ethyl-2H-benzo[b][1,4]oxazin-3(4H)-one](/CAS/GIF/90921-75-8.gif)
90921-75-8
28 suppliers
$55.00/250mg
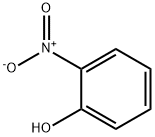
88-75-5
12 suppliers
$15.00/5mg
![2-Ethyl-2H-benzo[b][1,4]oxazin-3(4H)-one](/CAS/GIF/90921-75-8.gif)
90921-75-8
28 suppliers
$55.00/250mg

95-55-6
539 suppliers
$5.00/5G
![2-Ethyl-2H-benzo[b][1,4]oxazin-3(4H)-one](/CAS/GIF/90921-75-8.gif)
90921-75-8
28 suppliers
$55.00/250mg