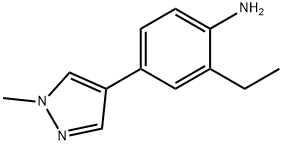
2-ethyl-4-(1-methyl-1H-pyrazol-4-yl)benzenamine synthesis
- Product Name:2-ethyl-4-(1-methyl-1H-pyrazol-4-yl)benzenamine
- CAS Number:1449669-06-0
- Molecular formula:C12H15N3
- Molecular Weight:201.27
45762-41-2
127 suppliers
$6.00/1g

847818-55-7
161 suppliers
$21.21/1gm:

1449669-06-0
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;caesium carbonate in 1,4-dioxane;water at 95; for 24 h;Inert atmosphere;
Steps:
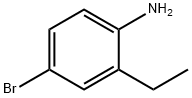
i Step i) 2-Ethyl-4-(l -methyl- lH-pyrazol-4-yl) aniline
-Bromo-2-ethylaniline (0.5 g, 2.5 mmol), 1 -methyl- 1 H-pyrazol-4-ylboronic acid (0.38 g, 3 mmol) and cesium carbonate (2.4 g, 7.5 mmol) were suspended in 1,4-dioxane (10 mL) and water (2 rriL) and the reaction mixture was then degassed (N2) for 5 min . After which [ Ι , - bis(diphenylphosphino)ferrocene]dichloropalladium(II) (0.102 g, 0.125 mmol) was added and the reaction mixture was heated to 95°C for 1 d. The reaction was cooled to room temperature and filtered through Celite, washed through with DCM. and the organics were washed with water, the layers were separated with the aqueous layer further extracted with DCM. The organics were combined, dried (hydrophobic filter) and concentrated in vacuo. The resulting residue was dissolved in DCM and allowed to load under gravity onto a 10 g SCX column, washed with DCM and MeOH and eluted with 7 N NH3 solution in MeOH : MeOH (1 :5). The eluent was concentrated in vacuo and the resulting residue was purified using column chromatography on silica gel and eluting with 0 - 75% EtOAc in isohexanes. The fractions containing product were combined and concentrated in vacuo to give the desired compound.
References:
WO2013/117645,2013,A1 Location in patent:Paragraph 00434