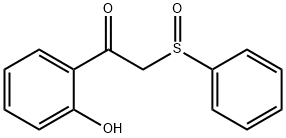
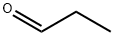2-Ethylchromone synthesis
- Product Name:2-Ethylchromone
- CAS Number:14736-30-2
- Molecular formula:C11H10O2
- Molecular Weight:174.2
Yield:14736-30-2 81 %
Reaction Conditions:
with piperidine;acetic acid in toluene at 50;
Steps:
Chemistry for synthesis of 7a~7z, 8 and 9 as follows:
General procedure: To a solution of 5a (0.26 g, 1.00 mmol) and 6a (0.13 g, 1.20 mmol) in toluene (2.00 mL), was added AcOH (11.2 μL, 0.20 mmol) and piperidine (19.8 μL, 0.20 mmol). The reaction mixture was stirred at 50 °C for 1 h, then quenched with H2O (2.00 mL) at room temperature and extraction with EtOAc. The combined organic layers were washed with brine (10 mL), dried with Na2SO4, filtered and concentrated under reduced pressure. The crude product was recrystallized with EtOAc and petroleum to give 7a as a yellow solid.
References:
Tang, Mei-Lin;Ning, Jin-Feng;Li, Yu-Hui;Zhang, Heyanhao;Liu, Mi;Dong, Ye-Jun;Chang, Jun [Tetrahedron Letters,2023,vol. 116,art. no. 154336] Location in patent:supporting information
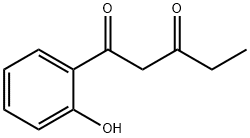
35115-15-2
0 suppliers
inquiry

14736-30-2
10 suppliers
inquiry
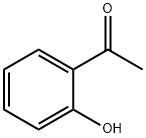
118-93-4
550 suppliers
$10.00/25g

14736-30-2
10 suppliers
inquiry

610-94-6
298 suppliers
$13.00/25g

14736-30-2
10 suppliers
inquiry