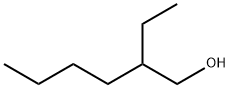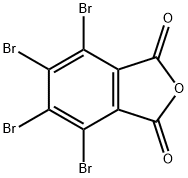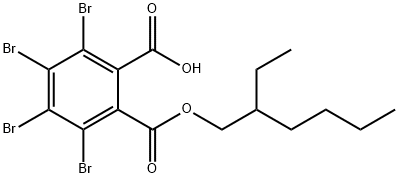
2-Ethylhexyl TetrabroMophthalate synthesis
- Product Name:2-Ethylhexyl TetrabroMophthalate
- CAS Number:61776-60-1
- Molecular formula:C16H18Br4O4
- Molecular Weight:593.93
Yield:-
Reaction Conditions:
with sodium carbonate at 20 - 120; for 3 h;Product distribution / selectivity;
Steps:
I
EXAMPLE I; SYNTHESIS OF 2-ETHYLHEXYLTETRABROMOBENZOATE ESTER FROM TETRABROMOPHTHALIC ANHYDRIDE ACCORDING TO A PRIOR ART BATCH PROCESS; In this example, 2-ethylhexyltetrabromobenzoate ester was produced using the known batch process taught in U. S. Patent No. 5,637, 757 as described below. The reactants and their quantities used in the batch process are listed below in Table 1. The results are illustrated in Table 2; The reactants listed in Table 1 were charged into a glass lined reactor under agitation. The mixture was heated to 120°C over a 1 hour period and then held at 120°C for 2 hours. The resulting intermediate was essentially the 2-ethylhexyl HALF-ESTER OF TETRABROMOPHTHALIC anhydride dissolved in excess 2-ethylhexanol. Then, a portion of this mixture was then transferred to another glass lined reactor. The mixture was heated to 200°C over a 1 hour period and then held at 200°C for 8 hours. The water of the reaction (generated by the formation of the undesired tetrabromophthalate diester) was separated from the reaction product. At the end of 8 hours the reaction product was cooled and washed with water to remove the catalyst. The excess 2-ethylhexanol was striped off under vacuum at pressures of between about 3 mmHg and 7 mmHg to yield a clear amber liquid product, which was tested using High Performance Liquid Chromatography (HPLC) and the Gardner Color Test (ASTM D1544-98 Standard Test Method for Color of Transparent Liquids). The results of these tests are shown in Table 2 below
References:
PABU SERVICES, INC. WO2005/16863, 2005, A1 Location in patent:Page/Page column 16-17