
(2-fluoro-3-iodophenyl)methanol synthesis
- Product Name:(2-fluoro-3-iodophenyl)methanol
- CAS Number:307975-02-6
- Molecular formula:C7H6FIO
- Molecular Weight:252.02

447464-03-1
99 suppliers
inquiry

307975-02-6
17 suppliers
inquiry
Yield:307975-02-6 97%
Reaction Conditions:
Stage #1: 2-fluoro-3-iodobenzoic acidwith Trimethyl borate in tetrahydrofuran at 0;
Stage #2: with borane dimethyl sulfide complex in tetrahydrofuran at 0 - 20;
Stage #3: with methanol in tetrahydrofuran;
Steps:
22.c c)
Trimethyl borate (1.92 mL, 17.2 mmol) was added dropwise to a stirred solution of 2-fluoro-3-iodobenzoic acid (preparation 22b, 4.39 g, 16.5 mmol) in tetrahydrofuran (25 mL) at 0 °C and the mixture was stirred for a further 15 minutes at this temperature. Then borane-methyl sulfide complex (10 M, 4.4 mL, 44 mmol) in tetrahydrofuran (3 mL) was added dropwise to the mixture at 0 °C and the mixture was then allowed to warm to room temperature. After 1 hour, the mixture was carefully quenched by dropwise addition of methanol (10 mL). After stirring overnight, the mixture was concentrated in vacuo and ethyl acetate was added to the residue. The organic layer was washed with saturated aqueous potassium carbonate solution, brine, dried (MgSO4) and evaporated to give the title compound (4.03 g, 97%) as an oil. 1H-NMR δ (CDCl3): 1.85 (t, J=6.0 Hz, 1H), 4.78 (d, J=6.0 Hz, 2H), 6.93 (t, J=9.0 Hz, 1H), 7.39-7.44 (m, 1H), 7.66-7.71 (m, 1H).
References:
EP2113503,2009,A1 Location in patent:Page/Page column 32-33

211943-27-0
54 suppliers
inquiry

307975-02-6
17 suppliers
inquiry
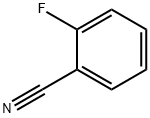
394-47-8
461 suppliers
$5.00/5g

307975-02-6
17 suppliers
inquiry
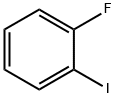
348-52-7
292 suppliers
$6.00/5g

307975-02-6
17 suppliers
inquiry