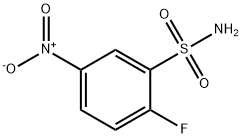
2-Fluoro-5-nitrobenzenesulfonamide synthesis
- Product Name:2-Fluoro-5-nitrobenzenesulfonamide
- CAS Number:881823-44-5
- Molecular formula:C6H5FN2O4S
- Molecular Weight:220.18
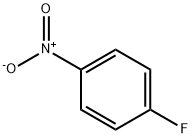
350-46-9
516 suppliers
$10.60/1gm:

881823-44-5
17 suppliers
inquiry
Yield:881823-44-5 51%
Reaction Conditions:
Stage #1: 4-Fluoronitrobenzenewith chlorosulfonic acid at 110; for 16 h;
Stage #2: with ammonia in water;ethyl acetate at 0; for 2 h;
Steps:
Synthesis of 2-fluoro-5-nitrobenzenesulfonamide (Compound 104-01)
A solution of 1-fluoro-4-nitrobenzene (compound 106) (1 g, 7.09 mmol, 1.0 eq) in chlorosulphonic acid (4 mL, 4 vol) was heated at 110° C. for 16 h.
The reaction mixture was cooled to rt and quenched with Sat.
NaHCO3 solution and extracted with diethyl ether (2*100 mL).
The combined organic layers were dried over sodium sulphate and concentrated to afford crude.
The crude was dissolved in EtOAc (40 mL) and aq.
Ammonia (40 mL) was added dropwise at 0° C., stirred for 2 h at same temperature.
After completion of reaction by TLC, the reaction mixture was diluted with water and extracted with EtOAc (2*100 mL).
The combined organic layers were dried over sodium sulphate and concentrated to afford 2-fluoro-5-nitrobenzenesulfonamide (compound 104-01) as a brown solid (800 mg, yield: 51%). TLC system: EtOAc: Hexane (50:50), Rf value: ?0.4; LCMS (m/z): 219.0 (M-H)+; 1H NMR (400 MHz, DMSO-d6) 8.56-8.52 (m, 2H), 8.06 (s, 2H), 7.78-7.74 (m, 1H).
References:
US2020/39979,2020,A1 Location in patent:Paragraph 0325-0326