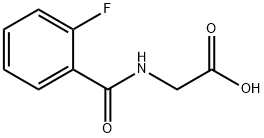
(2-FLUORO-BENZOYLAMINO)-ACETIC ACID synthesis
- Product Name:(2-FLUORO-BENZOYLAMINO)-ACETIC ACID
- CAS Number:363-34-8
- Molecular formula:C9H8FNO3
- Molecular Weight:197.16
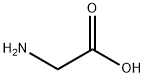
56-40-6
1204 suppliers
$5.00/25g
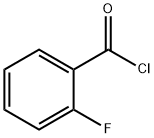
393-52-2
383 suppliers
$10.00/25g

363-34-8
28 suppliers
$55.00/250mg
Yield:363-34-8 27%
Reaction Conditions:
with sodium hydroxide at 0; for 1 h;
Steps:
3
Glycine (3.75 g, 0 . 05 μM) dissolved in sodium hydroxide (6 g, 0.15 μM) solution, ice water bath cooling to 0°C, adds by drops the neighbouring fluorine benzoyl chloride (6 ml, 0 . 05 μM), stirring for 1 hour, the reaction solution to room temperature after the restoration, adding hydrochloric acid solution to a large number of white solid separated out, the toner PH to 2 the left and right, filtering out solid, solid water after washing several times drying into a white powder solid neighbouring fluorine benzoyl glycine (2.64 g, 27%). neighbouring fluorine benzoyl glycine (1.93 g, 0 . 01 μM), anhydrous sodium acetate (0.82 g, 0 . 01 μM), benzaldehyde (1.02 ml, 0 . 01 μM) in three mixture, adding acetic anhydride 1 ml, in 100 °C the reflux condensation reaction under the oil bath 4 h, dichloromethane is used for extraction, the organic layer using saturated salt water, saturated sodium bicarbonate solution is washed, dried with anhydrous sodium sulfate, filtered, reduced pressure to remove the solvent to obtain the green solid (Z)-4 - benzylidene-2-(2-fluorophenyl)-oxazole-5(4H)-ketone (2.43 g, 89%). (Z)-4-benzylidene-2-(2-fluorophenyl)-oxazole-5(4H)-one (2.4 g, 9 mmol) first with 5 ml CHCl after dissolving, in the ice water bath cooling to 0 °C, dropwise 10 ml of chloroform for L - phenylalaninol (1.359 g, 9 mmol), the reaction 8 h after, dichloromethane extraction, the organic layer using saturated salt water washed, dried with anhydrous sodium sulfate, filtered, reduced pressure to remove the solvent to obtain a yellow solid (1.504 g, 40%).
References:
CN107011206,2017,A Location in patent:Paragraph 0093
![ETHYL 2-[(2-FLUOROBENZOYL)AMINO]ACETATE](/StructureFile/ChemBookStructure3/GIF/CB7756610.gif)
304657-05-4
20 suppliers
$112.00/1g

363-34-8
28 suppliers
$55.00/250mg

393-52-2
383 suppliers
$10.00/25g

363-34-8
28 suppliers
$55.00/250mg