2H-1,3-Benzoxazine-2,4(3H)-dione synthesis
- Product Name:2H-1,3-Benzoxazine-2,4(3H)-dione
- CAS Number:2037-95-8
- Molecular formula:C8H5NO3
- Molecular Weight:163.13
Yield:2037-95-8 97.9%
Reaction Conditions:
in N,N-dimethyl-formamide at 0 - 20;
Steps:
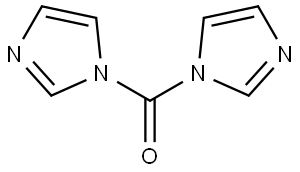
1 Example 1 Preparation of Compound 1 (2H-Benzo[e][1,3]oxazine-2,4(3H)-dione)
Add 20.0 g (0.146 mol) and 80 ml of salicylamide to a 250 ml reaction flask with a magnet and a thermometer.DMF, stirring and cooling to 0 to 5 ° C, 28.4 g (0.175 mol) of N'N-carbonyldiimidazole was added in portions, and the temperature was controlled at 0 to 5 °C. Then, the reaction was carried out at room temperature for 2 to 3 hours, 240 ml of purified water was added, pH was adjusted to 1 to 3 with 6 M hydrochloric acid aqueous solution, stirring was continued for 30 min, and filtration was carried out. The filter cake was rinsed with purified water, and the filter cake was placed in a blast drying oven at 45 . Drying at 50 ° C for 16 h gave 23.3 g of a white solid.The yield was 97.9%.
References:
CN108689876,2018,A Location in patent:Paragraph 0018; 0024-0025

65-45-2
536 suppliers
$5.00/25g
105-58-8
458 suppliers
$15.00/25g

2037-95-8
202 suppliers
$5.00/25mg

24424-99-5
840 suppliers
$13.50/25G

65-45-2
536 suppliers
$5.00/25g

2037-95-8
202 suppliers
$5.00/25mg