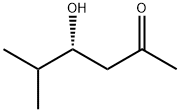
2-Hexanone, 4-hydroxy-5-methyl-, (4R)- (9CI) synthesis
- Product Name:2-Hexanone, 4-hydroxy-5-methyl-, (4R)- (9CI)
- CAS Number:181185-40-0
- Molecular formula:C7H14O2
- Molecular Weight:130.18
Yield:181185-40-0 95%
Reaction Conditions:
with C26H25NO2S at 0 - 20; for 24 h;optical yield given as %ee;enantioselective reaction;Aldol addition;
Steps:
4.2. General procedure for the asymmetric direct aldol reaction
General procedure: A round-bottomed flask was charged with catalyst 1 (0.2 mmol) and acetone (25 mL). The mixture was cooled to 0 °C, after which the corresponding aldehyde (1 mmol) was added and the mixture was stirred at room temperature for 24 hours. After this time, acetone was evaporated and the crude mixture was purified via column chromatography (hexane: ethyl acetate in gradient) to obtain optically active products 3a-f. The yields, specific rotations, and enantiomeric excess values are shown in Table 1 and Table 2.
References:
Rachwalski, Michal;Lesniak, Stanislaw;Kielbasinski, Piotr [Tetrahedron Asymmetry,2011,vol. 22,# 12,p. 1325 - 1327] Location in patent:experimental part
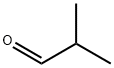
78-84-2
363 suppliers
$10.00/25mL

67-64-1
6 suppliers
$19.10/10ml
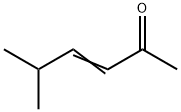
5166-53-0
66 suppliers
$87.00/25mL

181185-40-0
2 suppliers
inquiry