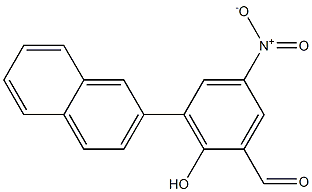
2-Hydroxy-3-(naphthalen-2-yl)-5-nitrobenzaldehyde synthesis
- Product Name:2-Hydroxy-3-(naphthalen-2-yl)-5-nitrobenzaldehyde
- CAS Number:1197212-87-5
- Molecular formula:C17H11NO4
- Molecular Weight:293.2735

16789-84-7
130 suppliers
$9.00/250mg
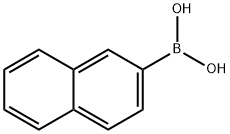
32316-92-0
468 suppliers
$6.00/1g

1197212-87-5
4 suppliers
inquiry
Yield:1197212-87-5 16%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,2-dimethoxyethane;ethanol;water at 80; for 6 h;
Steps:
3
Example 3Synthesis of 3-(2-naphthyl)-2-hydroxy-5-nitro-benzaldehyde - compound 7 1 6 73-Bromo-2-hydroxy-5-nitro-benzaldehyde (1 ) (2.Og, 0.008 mole) and 2-naphthyl boronic acid (6) (1.68g, 0.0097 mole) were dissolved in 30ml of 1 ,2-dimethoxyethane (DME). Ethanol (20 ml) and water (4ml) was added to the above reaction mixture followed by the addition of Na2CC>3 (3.44g, 0.0325 mole). The reaction mixture was stirred at 800C for 6h. Reaction mixture was cooled to room temperature and acidified with dilute ice cold hydrochloric acid and extracted with ethyl acetate. The organic layer was dried over dry sodium sulphate and concentrated to get solid. This solid was recrystallized using dichloromethane: EtOH (80:20) to obtain pure compound 7. Yield=0.380g (16%) M. P. = 193-201 0C1H NMR (CDCI3) δ (ppm) = 12.2 (s, 1 H), 10.07 (s, 1 H), 8.62 (d, J=17.94 Hz, 2H), 7.77-8.09 (m, 7H)
References:
WO2009/141236,2009,A1 Location in patent:Page/Page column 8