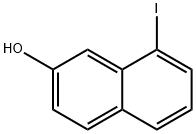
2-Hydroxy-8-iodonaphthalene synthesis
- Product Name:2-Hydroxy-8-iodonaphthalene
- CAS Number:29921-51-5
- Molecular formula:C10H7IO
- Molecular Weight:270.07
Yield: 47.7%
Reaction Conditions:
Stage #1:8-amino-2-naphthol with hydrogenchloride;sodium nitrite in water at 0; for 2 h;
Stage #2:potassium iodide in water at 60 - 70; for 1 h;
Steps:
2-hydroxy-8-iodonaphthalene (38).
A cold solution of NaNO2 (260 mg, 3.77 mmol) in water (1 ml) was slowly added to a solution of 2-hydroxy-1-naphthylamine (500 mg, 3.14mmol) in conc. HCl (0.3 ml) and water (1 ml) at 0 °C and the reaction mixture was stirred 2hrs at 0 °C. When diazotization was complete, a hot solution of KI (935.5 mg, 5.65 mmol) inwater (1.5 ml) at 60 °C was added slowly and suspension was heated at 70 °C for 1 hr. Thereaction mixture was basified with 1 N NaOH and ethyl acetate was added. The organic layerwas dried over anhydrous MgSO4, filtered and concentrated in vacuo. The residue waspurified by column chromatography on silica gel with nhexane/ethyl acetate (4:1) as theeluent to obtain the product (47.7 %, yellow solid). 1H - NMR (400 MHz, CDCl3) δ 8.02 (d,J = 7.3 Hz, 1H), 7.75 (d, J = 8.1 Hz, 1H), 7.69 (d, J = 8.8 Hz, 1H), 7.44 (d, J = 2.3 Hz, 1H),7.12 (ddd, J = 8.8, 2.4, 2.4 Hz, 1H), 7.04 (dd, J = 8.0, 7.5 Hz, 1H), 5.15 (s, 1H)
References:
Oh, Soo-Jin;Hwang, Seok Jin;Jung, Jonghoon;Yu, Kuai;Kim, Jeongyeon;Choi, Jung Yoon;Hartzell, H. Criss;Roh, Eun Joo;Justin Lee [Molecular Pharmacology,2013,vol. 84,# 5,p. 726 - 735] Location in patent:supporting information
118-46-7
224 suppliers
$10.00/1g

29921-51-5
18 suppliers
inquiry
