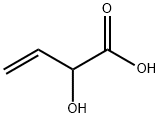
2-HYDROXY-BUT-3-ENOIC ACID ALLYL ESTER synthesis
- Product Name:2-HYDROXY-BUT-3-ENOIC ACID ALLYL ESTER
- CAS Number:98272-42-5
- Molecular formula:C7H10O3
- Molecular Weight:142.15
Yield:98272-42-5 72%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium carbonate in N,N-dimethyl-formamide at 45; for 4 h;Inert atmosphere;Schlenk technique;
Steps:
Allyl 2-hydroxybut-3-enoate (9)
To a solution of 84 (306 mg, 3.00 mmol), K2CO3 (435 mg, 3.15 mmol) and TBAI (221 mg, 0.60 mmol) in DMF (4 mL) was added allyl bromide (1.00 mL, 12.3 mmol) and the reaction mixture was stirred for 4 h at 45 °C. After cooling to ambient temperature, the reaction mixture was diluted with aq. HCl (1 M, 7.5 mL) and EtOAc (50 mL). The aqueous phase was extracted with EtOAc (4 times 20 mL). The combined organic phases were washed with water, sat. aq. NaHCO3 solution, water and brine. After drying with Na2SO4 the solvent was removed in vacuu (max. 50 mbar). The residue was dry-loaded on silica gel using CH2Cl2 and chromatographed on silica (pentane/diethyl ether 4:1to 2:1) to give 9 (310 mg, 2.16 mmol, 72%); colorless oil.
References:
Schmidt, Bernd;Aud?rsch, Stephan;Kunz, Oliver [Synthesis,2016,vol. 48,# 24,art. no. SS-2016-T0430-OP,p. 4509 - 4518] Location in patent:supporting information