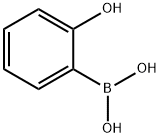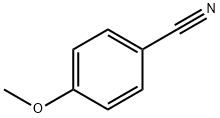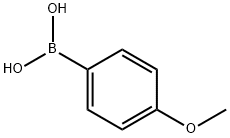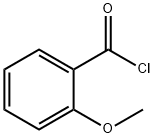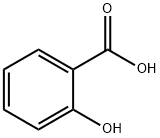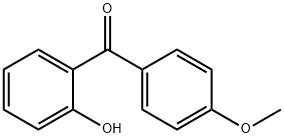
(2-hydroxyphenyl)-(4-methoxyphenyl)methanone synthesis
- Product Name:(2-hydroxyphenyl)-(4-methoxyphenyl)methanone
- CAS Number:18733-07-8
- Molecular formula:C14H12O3
- Molecular Weight:228.24
Yield:18733-07-8 95%
Reaction Conditions:
with trifluorormethanesulfonic acid;palladium diacetate;2-(3,5-dimethyl-1H-pyrazol-1-yl)pyridine in water at 90; for 1.5 h;
Steps:
General procedure for addition of arylboronic acids to nitrile
General procedure: To a mixture of arylboronic acid (1.2 mmol), nitrile (1.0 mmol), Pd(OAc)2 (4 mol%)and L1 (4 mol%), H2O (1.2 mL) and triflic acid (0.4 mL) were added and stirred at 60 °Cunder air for desired time (TLC monitoring). Then the reaction mixture was neutralized withsaturated NaHCO3 solution and extracted with ether. The combined ether solution waswashed with brine, dried by Na2SO4 and concentrated. The residue was purified by flashcolumn chromatography on silica gel using petroleum ether/cetone or petroleum ether/DCMas eluent to give the desired product.
References:
Das, Tuluma;Chakraborty, Amarnath;Sarkar, Amitabha [Tetrahedron Letters,2014,vol. 55,# 52,p. 7198 - 7202] Location in patent:supporting information
5449-69-4
36 suppliers
$60.00/100mg

18733-07-8
3 suppliers
inquiry