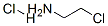
2-(1H-Imidazol-1-yl)ethanamine synthesis
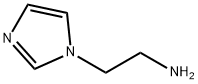
- Product Name:2-(1H-Imidazol-1-yl)ethanamine
- CAS Number:5739-10-6
- Molecular formula:C5H9N3
- Molecular Weight:111.15

Yield:5739-10-6 34%
Reaction Conditions:
with sodium hydroxide;tetra(n-butyl)ammonium hydrogensulfate in acetonitrile; for 21 h;Heating / reflux;
Steps:
70.a
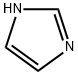
Imidazole (8.10 g, 119 mmol), 2-chloroethylamine monohydrochloride (15.2 g, 131 mmol), tetrabutylammonium hydrogensulfate (1.62 g, 4.8 mmol) and sodium hydroxide (17.1 g, 428 mmol) were combined with 100 ml_ acetonitrile and heated under reflux for 21 h. The reaction mixture was cooled and filtered. The filtrate was concentrated to a pale yellow oil. Flash chromatography (silica gel, gradient elution of acetonitrile to 9:1 acetonitrile/NH4OH) afforded 4.52 g (34%) of [2-(1H-imidazol-1-yl)ethyl]amine as a pale yellow oil. 1H NMR (DMSO-c/6): 5 7.58 (s, 1H), 7.17 (s, 1H), 6.83 (s, 1H), 3.86 (t, 2H), 2.79 (t, 2H), 2.10 (brs, 2H).
References:
WO2006/20415,2006,A1 Location in patent:Page/Page column 154
![Carbamic acid, [2-(1H-imidazol-1-yl)ethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/558441-67-1.gif)
558441-67-1
3 suppliers
inquiry

5739-10-6
82 suppliers
inquiry
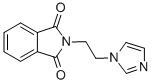
72459-53-1
10 suppliers
inquiry

5739-10-6
82 suppliers
inquiry
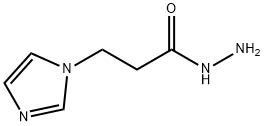
1190617-85-6
5 suppliers
inquiry

5739-10-6
82 suppliers
inquiry