
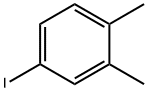
2-Iodo-4,5-dimethylnitrobenzene synthesis
- Product Name:2-Iodo-4,5-dimethylnitrobenzene
- CAS Number:39763-72-9
- Molecular formula:C8H8INO2
- Molecular Weight:277.06
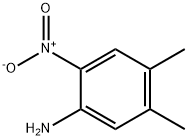
6972-71-0
171 suppliers
$6.00/1g

39763-72-9
9 suppliers
inquiry
Yield:39763-72-9 93.2%
Reaction Conditions:
Stage #1: 2-nitro-4,5-dimethylanilinewith sulfuric acid in lithium hydroxide monohydrate at 90; for 1 h;
Stage #2: with carbamide;potassium iodide;NaNO2 at 0 - 20; for 1.5 h;
Steps:
1.4; 2.5 (4) diazotization iodination:
168.2g of raw material 4,5-dimethyl-2-nitroaniline (purity 98.3%) was added to 1080ml of 10ωt.% sulfuric acid solution, the temperature was raised to 80-100°C, preferably 90°C, and stirred for 1 hour. Then it was cooled to 0°C, 252.0g NaNO2 (purity 30%), 6.08g urea (purity 99%) were added dropwise, and stirred; then the above reaction solution was slowly dropped into 700ml 30ωt.% KI solution at 0°C, and the reaction was carried out at room temperature. 1.5h. Add 47.6g Na2S2O3 and stir the reaction solution to form a yellow slurry,Suction filtration, the filter cake was washed three times with water (150 ml/time), and dried by blowing at 70° C. to obtain 277.4 g of the product 2-iodo-4,5-dimethylnitrobenzene. The yield of the product was 93.2%, and the purity was 92.7%.
References:
CN114591180,2022,A Location in patent:Paragraph 0048; 0052; 0058; 0062

2198-54-1
126 suppliers
$14.00/1g

39763-72-9
9 suppliers
inquiry
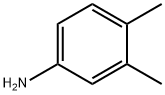
95-64-7
235 suppliers
$5.00/5G

39763-72-9
9 suppliers
inquiry
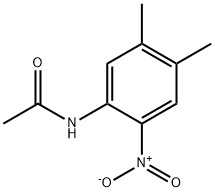
6970-77-0
35 suppliers
$28.60/250mg

39763-72-9
9 suppliers
inquiry
31599-61-8
172 suppliers
$6.00/1g

39763-72-9
9 suppliers
inquiry