
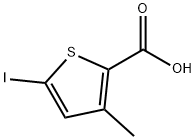
2-Iodo-4-methylthiophene synthesis
- Product Name:2-Iodo-4-methylthiophene
- CAS Number:16488-58-7
- Molecular formula:C5H5IS
- Molecular Weight:224.06
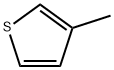
616-44-4
253 suppliers
$18.00/25g

16488-58-7
11 suppliers
inquiry
Yield:16488-58-7 73%
Reaction Conditions:
Stage #1: 3-Methylthiophenewith lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -25 - 0; for 1.08333 h;
Stage #2: with iodine in tetrahydrofuran;n-heptane;ethylbenzene at -15 - 10; for 2 h;
Steps:
1
Example 1 2-iodo-4-methyl-thiophene; A 3 L 4-Neck flask, equipped with overhead stirring, rubber septum, thermocouple and nitrogen inlet/outlet is charged with 3-methylthiophene (135 mL, 1.38 mol) and THF (1 L). The mixture is cooled to -200C and LDA (800 mL of Aldrich 2.5 M solution in THF/Heptane/ethylbenzene) is added via 12 gauge cannula over 35 minutes, adjusting the addition rate and cooling as necessary to maintain the reaction temp between -25°C and
References:
WO2008/121666,2008,A1 Location in patent:Page/Page column 8-9
854625-81-3
2 suppliers
inquiry

16488-58-7
11 suppliers
inquiry