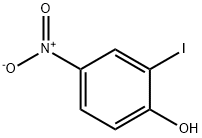
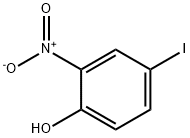
2-IODO-4-NITROPHENOL synthesis
- Product Name:2-IODO-4-NITROPHENOL
- CAS Number:89487-91-2
- Molecular formula:C6H4INO3
- Molecular Weight:265.01

99-57-0
12 suppliers
$20.89/5g

89487-91-2
85 suppliers
$82.00/250mg
Yield: 77%
Reaction Conditions:
Stage #1:2-hydroxy-5-nitroaniline with sulfuric acid;sodium nitrite in water;dimethyl sulfoxide at 0; for 1 h;Inert atmosphere;
Stage #2: with potassium iodide in water;dimethyl sulfoxide at 20; for 12 h;Inert atmosphere;
Steps:
2-Iodo-4-nitrophenol (11)
According to the modified procedures by Dai and Lai18) and Zhu et al.,19) to a solution of 2-amino-4-nitrophenol (10) (14.8 g, 96.0 mmol) in 30% H2SO4(500 mL) and dimethyl sulfoxide (DMSO) (500 mL) was added a solution of NaNO2 (9.94 g, 144 mmol) in water (50 mL) at 0°C. The reaction mixture was stirred at the same temperature for 1 h, after which a solution of KI (47.8 g, 288 mmol) in water (50 mL) was added. After stirring at room temperature(r.t.) for 1 h, another batch of KI (47.8 g, 288 mmol) in water(50 mL) was then added. The reaction mixture was stirred at r.t. for 12 h. After reaction was completed, the mixture was extracted with EtOAc, washed with brine and saturated aqueous solution of NaHSO3, dried over Na2SO4, filtered, and evaporated in vacuo. The residue was purified by flash column chromatography on silica gel eluting with hexane/EtOAc=1 : 1 to give 11 (19.7 g, 77%) as yellow crystals. mp 84-86°C(hexane/EtOAc); IR νmax: 3479, 3020, 1602, 1524, 1341 cm-1;1H-NMR (300 MHz, CDCl3) δ: 8.60 (d, J=2.4 Hz, 1H), 8.18(dd, J=9.0, 2.7 Hz, 1H), 7.07 (d, J=9.0 Hz, 1H), 6.23 (br s, 1H); 13C-NMR (75 MHz, CDCl3) δ: 160.4, 141.9, 134.4, 126.1, 114.6,84.5; HR-ESI-MS Calcd for C6H3INO3 [M-H]- 263.9163.Found 263.9164.
References:
Okitsu, Takashi;Ogasahara, Mizuki;Wada, Akimori [Chemical and Pharmaceutical Bulletin,2016,vol. 64,# 8,p. 1149 - 1153]

533-58-4
375 suppliers
$6.00/5g

89487-91-2
85 suppliers
$82.00/250mg
100-02-7
26 suppliers
$11.00/5G

89487-91-2
85 suppliers
$82.00/250mg

100-02-7
26 suppliers
$11.00/5G
305-85-1
106 suppliers
$65.00/1g

89487-91-2
85 suppliers
$82.00/250mg
540-38-5
341 suppliers
$10.00/1g
21784-73-6
52 suppliers
$75.00/500mg

89487-91-2
85 suppliers
$82.00/250mg

20294-48-8
3 suppliers
inquiry