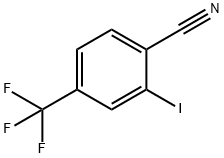
2-Iodo-4-trifluoromethyl-benzonitrile synthesis
- Product Name:2-Iodo-4-trifluoromethyl-benzonitrile
- CAS Number:1259323-42-6
- Molecular formula:C8H3F3IN
- Molecular Weight:297.02
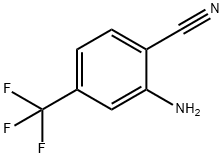
1483-54-1
133 suppliers
$10.00/1g

1259323-42-6
20 suppliers
inquiry
Yield:1259323-42-6 87%
Reaction Conditions:
with hydrogenchloride;potassium iodide;sodium nitrite in water;acetonitrile at 0 - 20;
Steps:
Potassium iodide (5.6 g, 34 mmol) and sodium nitrite (2.4 g, 35 mmol) were added to a solution of 2-amino-4-trifluoromethyl-benzonithle (2.58 g, 14 mmol) in acetonithle (60 ml_). The resultant mixture was cooled to 0 °C in an ice-water bath with magnetic stirring. Ice-cold concentrated HCI (14 ml_) was added slowly drop-wise to the reaction mixture, causing the reaction mixture to become cloudy and deep red in color. The reaction mixture was stirred at 0 °C for 30 minutes, then warmed to room temperature. Stirring at room temperature proceeded for 3 hours. The reaction mixture was poured into water, and the resulting suspension was extracted with ethyl acetate. The organic layer was dried (Na2SO4), filtered, and concentrated to give a purple liquid. A solution of this crude product and dichloromethane (200 ml_) was evaporated onto silica gel, and the dry silica gel-supported product was loaded onto a silica gel column. Manual flash chromatography using 5% ethyl acetate in hexanes afforded 2-iodo-4-trifluoromethyl-benzonithle as 3.58 g (87%) of a purple, crystalline solid.
References:
WO2010/55005,2010,A1 Location in patent:Page/Page column 74-75
349-03-1
206 suppliers
$8.00/5g

1259323-42-6
20 suppliers
inquiry
778-94-9
189 suppliers
$10.00/5g

1259323-42-6
20 suppliers
inquiry