2-IODOBENZYLZINC BROMIDE 0.5M IN THF synthesis
- Product Name:2-IODOBENZYLZINC BROMIDE 0.5M IN THF
- CAS Number:117269-71-3
- Molecular formula:C7H6BrIZn
- Molecular Weight:362.321
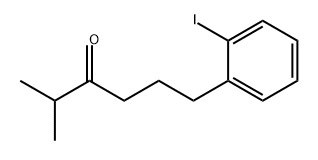
25183-76-0
2 suppliers
inquiry

117269-71-3
12 suppliers
$256.00/50ml
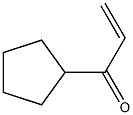
872834-99-6
0 suppliers
inquiry
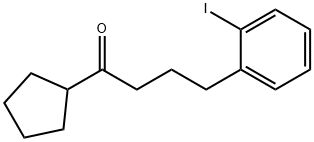
Yield:872834-99-6 90%
Reaction Conditions:
Stage #1: (2-iodobenzyl)zinc bromidewith copper(I) cyanide;lithium chloride in tetrahydrofuran at -78 - 25; for 0.5 h;
Stage #2: cyclopentylvinylketonewith chloro-trimethyl-silane in tetrahydrofuran at -78 - 20; for 4 h;
Steps:
1
Tetrahydrofuran (12.5 ml) was added to anhydrous lithium chloride (1.06 g, 25 mmol) and copper(l) cyanide (1.12 g, 12.5 mmoles) whilst stirring under an atmosphere of nitrogen at 25 °C. After stirring for 10 minutes this solution was cooled to -78 °C and 2-iodobenzylzinc bromide (25 ml, 0.5 M in THF, 12.5 mmoles) was added. The solution was then warmed to -15 °C for 20 minutes before re-cooling to -78 °C. In a second flask, cyclopentylzinc bromide (25 ml, 0.5 M in THF, 12.5 mmoles) was added to tetrakis(triphenylphosphine) palladium(O) (145 mg, 0.125 mmoles, 1 mol%) under nitrogen at 0 °C. After 5 minutes acryloyl chloride (1.1 ml, 13.5 mmoles) was added and the mixture was left to stir at 0° C for approximately 1 hour. To the first flask containing the copper/zinc complex, chlorotrimethylsilane (3.2 ml, 25.2 mmoles) was added (solution still at -78 °C) followed by the vinyl ketone in THF solution which was prepared in the second flask. Stirring was continued at -78 ° C for about 3 hours and then the reaction was warmed to room temperature and stirring continued for a further 1 hour. The reaction mixture was partitioned between water (100 ml) and diethyl ether (100 mL) and the aqueous layer was extracted with two further portions of ether (2 x 100 mL). The combined ether extracts were washed with brine (2 x 100 mL) and were dried over magnesium sulfate. The solvent was removed to give a crude product which after purification (silica column, 19:1 cyclohexaneiethyl acetate) gave the title compound as a colourless oil (3.84 g, 90%).1H-NMR: SH (CDCI3, 400 MHz) 7.82 (d, 1H), 7.32-7.18 (m, 2H), 6.94-6.85 (m, 1H), 2.88 (qn, 1H), 2.73 (t, 2H), 2.53 (t, 2H) and 2.0-1.45 (t, 10 H)
References:
WO2006/401,2006,A1 Location in patent:Page/Page column 33
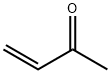
78-94-4
4 suppliers
$35.79/25ml

117269-71-3
12 suppliers
$256.00/50ml
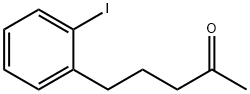
501346-70-9
0 suppliers
inquiry
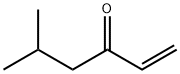
2177-32-4
21 suppliers
inquiry

117269-71-3
12 suppliers
$256.00/50ml
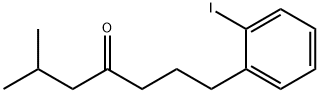
872835-46-6
0 suppliers
inquiry
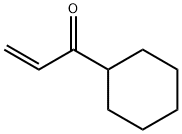
2177-34-6
13 suppliers
inquiry

117269-71-3
12 suppliers
$256.00/50ml
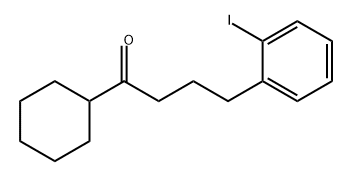
872835-08-0
0 suppliers
inquiry