
2-isobutylaniline synthesis
- Product Name:2-isobutylaniline
- CAS Number:71182-59-7
- Molecular formula:C10H15N
- Molecular Weight:149.23
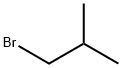
78-77-3
348 suppliers
$14.00/5g
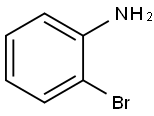
615-36-1
418 suppliers
$10.00/5g

71182-59-7
31 suppliers
$184.00/250mg
Yield:71182-59-7 74%
Reaction Conditions:
Stage #1: Isobutyl bromidewith magnesium in tetrahydrofuran at 55; for 1.58333 h;Inert atmosphere;
Stage #2: with zinc(II) chloride in tetrahydrofuran at 39; for 1.75 h;Inert atmosphere;
Stage #3: 2-bromoanilinewith dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane;palladium diacetate;trisodium Edetate in tetrahydrofuran;water at 32 - 33; for 0.5 h;Inert atmosphere;
Steps:
20.a Synthesis of 2-isobutylaniline
14.59 g (0.60 mol) of magnesium shavings are suspended under argon in 50 ml of tetrahydrofuran. 90.4 g (0.66 mol) of 1-bromo-2-methylpropane in 200 ml of tetrahydrofuran are slowly added during 45 minutes by carefully regulating the exothermy of the Grignard reaction by cooling with an ice-bath keeping the reaction temperature at a maximum of55°C. The grey suspension is further stirred during 50 minutes and allowed to cool down to room temperature, giving a grey-brown solution. A colorless solution of 40.89 g (0.30 mol) of anhydrous zinc chloride in 200 ml of tetrahydrofuran is added during 10 minutes and the released exothermy carefully regulated with an ice-bath keeping the temperature at a maximum of 39°C. The resulting grey thick suspension is further stirred during 95 minutesuntil the temperature reaches 26°C, and slowly added during 30 minutes to a red-brown solution of 26.3 g (150 mmol) of 2-bromoaniline, 1.31 g (3.00 mmol) of 2-dicyclohexylphosphino-2’ ,6’ -bis(N,N-dimethylamino)biphenyl (= CPhos), 0.34 g (1.51 mmol) of palladium(ll) acetate in 200 ml of THF, by carefully controlling the exothermy at a maximum of 32°C using a water-bath. 50 ml of water are first carefully added under coolingkeeping the temperature at a maximum of 33°C, followed by the addition of 500 ml of water and 200 ml of saturated ethylenediaminetetraacetic acid trisodium salt hydrate (EDTA-Na3), and by stirring for 30 minutes. The suspension is filtered through a layer of Hyflo filter aid and the filter aid rinsed with 500 ml of toluene. The organic phase is separated and washed with 100 ml of EDTA-Na3 and 100 ml of saturated sodium chloride, followed by drying oversodium sulfate and concentration under vacuum. The oil is further distilled (120°C, 0.1 mbar) giving the title product as a colorless oil (yield: 16.5 g (74%)).1HNMR (400 MHz, CD2CI2): = 1.00 (d, 6 H), 1.96 (dq, I H), 2.42 (d, 2 H), 3.64 (br. s,2 H),6.72 (m, 2 H), 7.04 (m, 2 H).
References:
WO2015/14835,2015,A1 Location in patent:Page/Page column 174

49660-91-5
3 suppliers
inquiry

71182-59-7
31 suppliers
$184.00/250mg
611-70-1
185 suppliers
$6.00/5g

71182-59-7
31 suppliers
$184.00/250mg
57991-54-5
13 suppliers
inquiry

71182-59-7
31 suppliers
$184.00/250mg

408538-58-9
1 suppliers
inquiry

71182-59-7
31 suppliers
$184.00/250mg