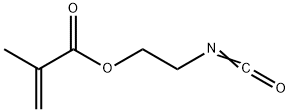
2-Isocyanatoethyl methacrylate synthesis
- Product Name:2-Isocyanatoethyl methacrylate
- CAS Number:30674-80-7
- Molecular formula:C7H9NO3
- Molecular Weight:155.15
![2-Propenoic acid, 2-methyl-, 2-[(1H-imidazol-1-ylcarbonyl)amino]ethyl ester](/CAS/20210305/GIF/89743-58-8.gif)
89743-58-8
1 suppliers
inquiry

30674-80-7
262 suppliers
$41.00/1g
Yield:-
Reaction Conditions:
Stage #1:2-(1H-imidazole-1-carboxamido)ethyl methacrylate with toluene-4-sulfonic acid in toluene at 23 - 30; for 3.5 h;
Stage #2: in toluene at 80; for 8 h;Product distribution / selectivity;
Steps:
4
Example 4: 2-Methyl-acrylic acid 2-isocvanato-ethyl ester; In a 1000 ml four neck roundbottom flask 35.5 g (200 mMole; 97 %) CDI and 22.3 mg phenothiazin were suspended in 200 ml ethyl acetate. The suspension was stirred for about 30 min at about 23°C. The temperature dropped to about 21 °C during that time and CDI dissolved partly. To the stirred suspension 61 g 2-(2-Methyl-acryloyloxy)-ethyl- ammonium; tosylate (200 mMole; 99%) were added in portions within about 30 min such that the temperature of the well agitated reaction mixture did not exceed about 30 °C. An off-white to brownish suspension was obtained that was stirred for another about 24 hours. The reaction mixture was filtered and the white precipitate washed with ethyl acetate. The solvent was distilled from the clear yellow to brown filtrate at about 40 °C under exclusion of light. A slightly viscous clear light yellow to brown resin was obtained that, according to proton NMR consisted of 2-Methyl-acrylic acid 2-[(imidazole-1- carbonyl)-amino]-ethyl ester and about 16 wt.-% ethyl acetate. 38.04 g (200 mMole) para- toluene sulfonic acid monohydrate were refluxed together with about 500 ml toluene using a Dean-Stark trap. 3.6 g water (100 % theory) had been separated after about 4 h. Thereafter, about 300 ml toulene were distilled off. To this solution a solution consisting of the above synthesized product dissolved in about 50 ml toluene was added within about 30 min such that the temperature of the reaction mixture did not exceed about 30 °C. The initially separating yellowish oil crystallized subsequently giving a whitish suspension after about 3 hours of stirring at about 23 °C. The suspension was heated to about 80 °C and kept at that temperature for about 3 h. The precipitate disappeared and imidazolium tosylate was formed within about 15 min. The suspension was kept for another about 5 hours at that temperature. The reaction mixture was filtered and the white precipitate washed with about 50 ml toluene. The solvent was removed from the yellow filtrate at about 40 °C at about 50 mbar. Proton NMR indicates that the clear yellow liquid consisted of MOI, toluene and residual uncleaved 2-Methyl-acrylic acid 2-[(imidazole-1 -carbonyl)- amino]-ethyl ester. The product was fractionated under vacuum. At about 43 °C and about 1 mbar 20g product was obtained (64 % of theory). The clear colorless liquid had a purity of 98 % MOI (GC). The residual chlorine content found was 3 ppm.
References:
3M INNOVATIVE PROPERTIES COMPANY;STEIGER, Wolf,;BISSINGER, Peter, WO2011/130032, 2011, A1 Location in patent:Page/Page column 23