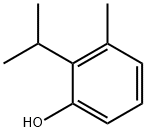
2-isopropyl-m-cresol synthesis
- Product Name:2-isopropyl-m-cresol
- CAS Number:3228-01-1
- Molecular formula:C10H14O
- Molecular Weight:150.22

1412-97-1
0 suppliers
inquiry

3228-01-1
2 suppliers
inquiry
Yield:3228-01-1 98%
Reaction Conditions:
with platinum(IV) oxide;hydrogen in ethyl acetate at 10; under 775.743 Torr; for 6 h;
Steps:
301.B Step B. 2-i sopropyl-3 -methylphenol .
To a solution of 3 -methyl-2-(prop- 1 -en -2- yl)phenol (3 g, 20.2 mmol, 1.0 eq) in ethyl acetate (30 mL) was added PtCh (460 mg, 2.02 mmol, 0.1 eq) under N2. The suspension was degassed under vacuum and purged with H2 several times. The mixture was stirred under H2 (15 psi) at 10 °C for 6 hours. The reaction mixture was filtered and the filtrate was concentrated to give the title compound (3 g, 98% yield). White solid. 1HNMR (400 MHz, chloroform-d) d = 6.95 (t, J= 8.0 Hz, 1H), 6.74 (d, J= 7.6 Hz, 1H), 6.56 (d, J= 8.0 Hz, 1H), 4.64 (s, 1H), 3.37 - 3.25 (m, 1H), 2.35 (s, 3H), 1.40 (s, 3H), 1.38 (s, 3H).
References:
WO2021/41671,2021,A1 Location in patent:Paragraph 01117
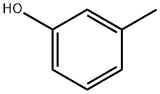
108-39-4
467 suppliers
$13.00/25g
67-63-0
1553 suppliers
$18.00/25ML
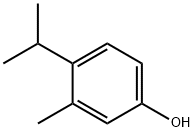
3228-02-2
263 suppliers
$8.00/5g
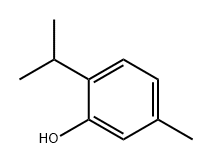
89-83-8
687 suppliers
$5.00/10g

3228-01-1
2 suppliers
inquiry

108-39-4
467 suppliers
$13.00/25g

67-63-0
1553 suppliers
$18.00/25ML

3228-02-2
263 suppliers
$8.00/5g

89-83-8
687 suppliers
$5.00/10g

3228-01-1
2 suppliers
inquiry
19177-04-9
20 suppliers
$45.00/1g

108-39-4
467 suppliers
$13.00/25g

3228-01-1
2 suppliers
inquiry