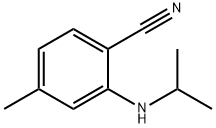
2-isopropylamino-4-methylbenzonitrile synthesis
- Product Name:2-isopropylamino-4-methylbenzonitrile
- CAS Number:28195-00-8
- Molecular formula:C11H14N2
- Molecular Weight:174.24
Yield:-
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);caesium carbonate;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in toluene at 90; for 6 h;Buchwald-Hartwig Coupling;
Steps:
1
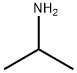
{4-methyl-2-[(propan-2-yl)amino]phenyl}(phenyl)methanaminium chloride - The titled compound was obtained following the modified procedure described in WO2006035157 (Protocol A). The starting material 2-(isopropylamino)-4- methylbenzonitrile was prepared from 2-bromo-4-methylbenzonitrile using a Ex.77 Buchwald-Hartwig reaction (2-bromo-4-methylbenzonitrile (1 eq.), isopropylamine (1 .5 eq.), BINAP (0.05 eq.), Pd2(dba)3 (0.03 eq.), Cs2C03 (2 eq.) in toluene at 90°C for 6h) - Yield: 43% ; appearance: pale yellow solid ; 1 H NMR, d (ppm) (Methanol d4) : 1 .06 (d, 3H, J=6.2Hz); 1.15 (d, 3H, J=6.2Hz); 2.25 (s, 3H); 3.51 -3.58 (m, 1 H); 5.88 (s, 1 H); 6.61 (s, 2H); 7.25-7.47 (m, 6H); 8.94 (br s, 3H)
References:
WO2016/102633,2016,A1 Location in patent:Page/Page column 59