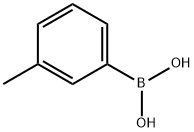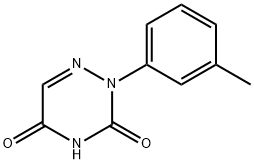
2-m-Tolyl-2H-[1,2,4]triazine-3,5-dione synthesis
- Product Name:2-m-Tolyl-2H-[1,2,4]triazine-3,5-dione
- CAS Number:55784-52-6
- Molecular formula:C10H9N3O2
- Molecular Weight:203.2
Yield:55784-52-6 80%
Reaction Conditions:
with pyridine;oxygen;copper diacetate in N,N-dimethyl-formamide at 20; for 2 h;Chan-Lam Coupling;
Steps:
General procedure for the synthesis of title compounds(3a-r):
General procedure: To a solution of 6-azauracil (100 mg, 0.88 mmol) inDMF (10.0 mL) was added base (1.76 mmol) and Cu(OAc) 2(159 mg, 0.88 mmol) at room temperature. The resulting reationmixture was degassed with oxygen for 10 min and then addedarylboronic acids (0.96 mmol) at room temperature and stirredat appropriate temperature (Table-1) under oxygen atmosphere.The reaction mixture was diluted with water (15 mL) andextracted with dichloromethane (3 × 15 mL). The organic layerwashed with H 2 O (15 mL), brine solution (15 mL), dried overNa 2 SO 4 and concentrated. The obtained crude product waspurified by column chromatography (0 to 10 % CH 3 OH/CH 2 Cl 2 )to afford the title compounds.
References:
Gulipalli, Kali Charan;Bodige, Srinu;Ravula, Parameshwar;Bolla, R. Sekhar;Endoori, Srinivas;Cherukumalli, Purna Koteswara Rao;Seelam, Nareshvarma [Asian Journal of Chemistry,2018,vol. 30,# 11,p. 2495 - 2501]