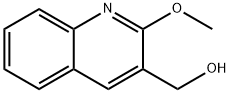
(2-methoxy-3-quinolinyl)methanol synthesis
- Product Name:(2-methoxy-3-quinolinyl)methanol
- CAS Number:1038969-22-0
- Molecular formula:C11H11NO2
- Molecular Weight:189.21

139549-06-7
47 suppliers
$22.00/250mg

1038969-22-0
8 suppliers
inquiry
Yield:1038969-22-0 100%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0;
Steps:
(2-methoxyquinolin-3-yl)methanol
(2-methoxyquinolin-3-yl)methanol
2-Methoxyquinoline-3-carbaldehyde (1.45 g, 7.75 mmol) was suspended in methanol (20 mL) and the mixture was cooled to 0° C. Sodium borohydride (600 mg, 15.86 mmol) was added, causing bubbling.
The reaction mixture was stirred and gradually warmed to room temperature overnight (let ice bath melt).
The reaction mixture was concentrated, and the crude material was taken up in saturated aqueous bicarbonate solution (50 mL) and extracted with dichloromethane (2*50 mL).
The combined organic layers were dried over Na2SO4, filtered, and concentrated to afford the title compound (1.46 g, 7.72 mmol, 100% yield).
1H NMR (500 MHz, DMSO-d6) δ ppm 8.19 (q, J=1.2 Hz, 1H), 7.90 (dd, J=8.0, 1.5 Hz, 1H), 7.82-7.72 (m, 1H), 7.62 (ddd, J=8.4, 6.9, 1.5 Hz, 1H), 7.42 (ddd, J=8.1, 6.9, 1.2 Hz, 1H), 5.44-5.30 (m, 1H), 4.66-4.54 (m, 2H), 4.01 (s, 3H); MS (ESI+) m/z 190 (M+H)+.
References:
US2018/99932,2018,A1 Location in patent:Paragraph 1511; 1521; 1798

67-56-1
739 suppliers
$9.00/25ml
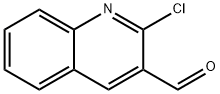
73568-25-9
186 suppliers
$9.00/250mg
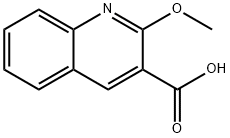
70659-29-9
18 suppliers
inquiry

1038969-22-0
8 suppliers
inquiry

139549-06-7
47 suppliers
$22.00/250mg

73568-25-9
186 suppliers
$9.00/250mg

1038969-22-0
8 suppliers
inquiry
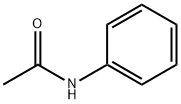
103-84-4
343 suppliers
$12.00/100g

1038969-22-0
8 suppliers
inquiry