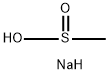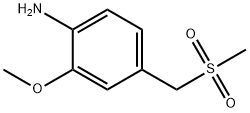
2-methoxy-4-[(methylsulfonyl)methyl]benzenamine synthesis
- Product Name:2-methoxy-4-[(methylsulfonyl)methyl]benzenamine
- CAS Number:895636-32-5
- Molecular formula:C9H13NO3S
- Molecular Weight:215.27
Yield:895636-32-5 83%
Reaction Conditions:
Stage #1: 4-(chloromethyl)-2-methoxy-1-nitrobenzene;sodium methansulfinate in ethanol at 80; for 16 h;
Stage #2: with hydrogen;palladium 10% on activated carbon in ethanol at 20; under 2585.81 Torr;
Steps:
B84.B
Step B/lntermediate B84: 2-(methyloxy)-4-[(methylsulfonyl)methyl]aniline; To a solution of 3-methoxy-4-nitrobenzyl chloride (2.76 g, 13.7 mmol) in 20 mL of absolute ethanol was added methanesulphinic acid sodium salt (1.0 g, 27.4 mmol). The reaction was heated to 800C for 16h. The reaction was cooled to room temperature and poured into water and the aqueous layer was extracted with ethyl acetate. Combined organics were dried over anhydrous MgSO4, filtered, concentrated onto silica gel and purified by flash chromatography with ethyl acetate/hexanes as the eluent. The fractions containing the desired product were concentrated to dryness, then redisolved in absolute ethanol and 10% Palladium on carbon (500 mg) was added and the reaction was placed on the Fischer-Porter hydrogenation apparatus and treated with 50 psi of H2 gas overnight to afford 2- (methyloxy)-4-[(methylsulfonyl) methyl]aniline (2.46g, 83%). 1H NMR (400 MHz,
References:
WO2009/20990,2009,A1 Location in patent:Page/Page column 122-123