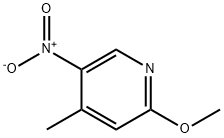
2-METHOXY-5-NITRO-4-PICOLINE synthesis
- Product Name:2-METHOXY-5-NITRO-4-PICOLINE
- CAS Number:6635-90-1
- Molecular formula:C7H8N2O3
- Molecular Weight:168.15
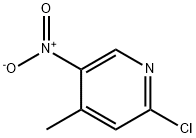
23056-33-9
331 suppliers
$3.00/1g

124-41-4
683 suppliers
$12.00/25g

6635-90-1
160 suppliers
$8.00/250mg
Yield: 98%
Reaction Conditions:
in methanol at 0 - 20;
Steps:
49 2-Methoxy-4-methyl-5-nitropyridine
Reference Example 49 2-Methoxy-4-methyl-5-nitropyridine A solution of 2-chloro-4-methyl-5-nitropyridine (3.0 g, 17.3 mmol) in methanol (20 mL) was added a solution of sodium methoxide (28% in methanol, 10 mL) dropwise at 0° C. The mixture was warmed to room temperature and stirred for 16 hr. The mixture was neutralized by addition of aqueous ammonium chloride and concentrated in vacuo. The residue was dissolved in water and extracted with ethyl acetate. Organic layer was washed with brine, dried over anhydrous magnesium sulfate and concentrated in vacuo to give the title compound (2.84 g, 16.8 mmol, 98%) as a yellow solid. 1H NMR (CDCl3) δ 2.62 (s, 3H), 4.01 (s, 3H), 6.64 (s, 1H), 8.94 (s, 1H). MS Calcd.: 168; Found: 169 (M+H).
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED US2009/186879, 2009, A1 Location in patent:Page/Page column 59

67-56-1
739 suppliers
$7.29/5ml-f

23056-33-9
331 suppliers
$3.00/1g

6635-90-1
160 suppliers
$8.00/250mg

23056-33-9
331 suppliers
$3.00/1g

6635-90-1
160 suppliers
$8.00/250mg
695-34-1
528 suppliers
$5.00/10g

6635-90-1
160 suppliers
$8.00/250mg
21901-41-7
281 suppliers
$6.00/1g

6635-90-1
160 suppliers
$8.00/250mg