
2-methoxy-5-nitropyrimidine synthesis
- Product Name:2-methoxy-5-nitropyrimidine
- CAS Number:14001-69-5
- Molecular formula:C5H5N3O3
- Molecular Weight:155.11
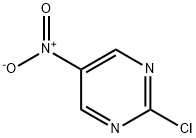
10320-42-0
354 suppliers
$7.00/250mg

124-41-4
682 suppliers
$12.00/25g

14001-69-5
71 suppliers
inquiry
Yield: 75%
Reaction Conditions:
in methanol for 1 h;Reflux;
Steps:
40
Example 40Synthesis of 4-[2-(difluoromethyl)-4-methoxy-1H-benzimidazol-1-yl]-N-(2-methoxy-5-pyrimidinyl)-6-(4-morpholinyl)-1,3,5-triazin-2-amineThe compound was synthesized according to Method A.To a solution of sodium methoxide (0.090 g of sodium) in MeOH (12 mL) was added 0.486 g (3.03 mmol) of 2-chloro-5-nitropyrimidine, and the mixture was heated under reflux for 1 hr. After cooling, the mixture was concentrated in vacuo, extracted with EtOAc, and washed with water. The aqueous layer was extracted with CHCl3 and the combined organic layers were dried (Na2SO4), and concentrated, to give 0.347 g (75% yield) of 2-methoxy-5-nitropyrimidine as a yellow powder: 1H NMR (CDCl3) δ9.31 (s, 2H), 4.17 (s, 3H); LCMS (APCI+) m/z: 156 (MH+, 100%).To 0.342 g (2.20 mmol) of the above nitro compound in MeOH (20 mL) was added 0.30 g of 10% Pd/C and the mixture was stirred under hydrogen (25 in/Hg) for 18 hrs. The reaction mixture was filtered through celite, and concentrated, to give 0.274 g (100% yield) of 5-amino-2-methoxypyrimidine as a colorless oil: 1H NMR (DMSO-d6) δ 8.05 (s, 2H), 3.94 (s, 3H); LCMS (APCI+) m/z: 126 (MH+, 100%).To 0.274 g (2.19 mmol) of the above amino compound in THF (3 mL) was added 1.25 mL of NaHMDS (2 M solution in THF) and the mixture was stirred for 10 min. A solution of 0.31 g (0.78 mmol) of 1-[4-chloro-6-(4-morpholinyl)-1,3,5-triazin-2-yl]-2-(difluoromethyl)-4-methoxy-1H-benzimidazole in THF (5 mL) was added and the resulting mixture was stirred for 90 min. The reaction mixture was neutralized with acetic acid, diluted with water, and extracted with EtOAc. The organic layer was washed with water and aq. NH3, dried, and concentrated. Recrystallization from EtOH/CH2Cl2 gave 0.098 g (26% yield) of 4-[2-(difluoromethyl)-4-methoxy-1H-benzimidazol-1-yl]-N-(2-methoxy-5-pyrimidinyl)-6-(4-morpholinyl)-1,3,5-triazin-2-amine: mp 255-258° C.; 1H NMR (DMSO-d6) 810.07 (s, 1H), 8.88-8.74 (m, 2H), 8.15-7.42 (m, 3H), 6.97 (d, J=8.0 Hz, 1H), 3.98 (s, 3H), 3.93 (s, 3H), 3.82-3.72 (m, 8H); Anal. Calcd. for C21H21F2N6O3: C, 52.0; H, 4.4; N, 26.0. Found: C, 52.1; H, 4.5; N, 26.0%.
References:
Pathway Therapeutics Limited US2011/9405, 2011, A1 Location in patent:Page/Page column 55-56
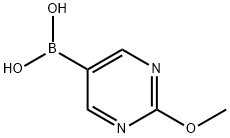
628692-15-9
221 suppliers
$6.00/250mg

14001-69-5
71 suppliers
inquiry

5329-33-9
102 suppliers
$65.00/5g
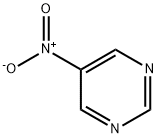
14080-32-1
30 suppliers
inquiry
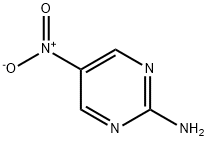
3073-77-6
273 suppliers
$7.00/250mg

14001-69-5
71 suppliers
inquiry