
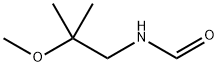
2-METHOXYISOBUTYL ISOCYANIDE synthesis
- Product Name:2-METHOXYISOBUTYL ISOCYANIDE
- CAS Number:109434-22-2
- Molecular formula:C6H11NO
- Molecular Weight:113.16
112129-25-6
13 suppliers
inquiry

109434-22-2
19 suppliers
inquiry
Yield: 80%
Reaction Conditions:
with triethylamine;trichlorophosphate in dichloromethane at -5 - 3;Product distribution / selectivity;
Steps:
7.1
Phosphorus oxychloride (from a fresh unopened bottle, 94 g, 0.61 mol) was added dropwise to a solution of MIBF (73 g, 99.7% (GC), 0.55 mol) and triethylamine (140.91 g, 1.39 mol) in anhydrous dichloromethane (555 mL) maintained between -5° C. and +3° C. The solution was stirred overnight at ice temperature. The reaction was quenched by pouring it carefully into a solution of sodium carbonate (123 g) in water (500 mL) cooled in an ice-water bath. The solution was stirred for 30 minutes in ice and diluted with water (500 mL). The precipitate was filtered off on Celite and rinsed portionwise with dichloromethane (925 mL). The organic layer was isolated from the filtrate. The remaining aqueous layer was extracted twice with dichloromethane (twice 275 mL). The organic layers were combined and dried over anhydrous sodium sulfate. The solution was filtered and concentrated by rotary evaporation to a volume of 250-300 mL. MIBI was isolated by distillation under reduced pressure (5-6 mmHg) as a colorless liquid (50.49 g, 80% yield, 99.7% (GC), b.p.: 46° C./5-6 mmHg). TLC Rf 0.6 (4:1 ethyl acetate/hexanes, detection with iodine stain) Rf 0.2 (CH2Cl2, detection with iodine stain); FTIR(neat): v 2983, 2944, 2833, 2152, 1470, 1388, 1370, 1294, 1242, 1184, 1166, 1079, 1010, 991, 945, 879, 857, 744 cm-1; 1H-NMR (600 MHz, CDCl3): δ 1.23 (s, 6H, 2 CH3), 3.21 (s, 3H, OCH3), 3.33 (m, 2H, 2J1H,14N=1.8 Hz, CH2); 13C-NMR(151 MHz, CDCl3): δ 22.4 (2 CH3), 49.9 (OCH3), 50.5 (t, 1J13C,14N=6.8 Hz, CH2), 73.2 (C), 157.6 (t, 1J13C,14N=5.3 Hz, N≡C).
References:
DRAXIS Specialty Pharmaceuticals Inc. US2008/102028, 2008, A1 Location in patent:Page/Page column 9; 11-12

89282-70-2
71 suppliers
$45.00/50mg

7732-18-5
460 suppliers
$12.69/100ml

109434-22-2
19 suppliers
inquiry

89282-70-2
71 suppliers
$45.00/50mg

109434-22-2
19 suppliers
inquiry