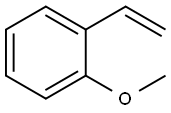
2-METHOXYSTYRENE synthesis
- Product Name:2-METHOXYSTYRENE
- CAS Number:612-15-7
- Molecular formula:C9H10O
- Molecular Weight:134.18
Yield:612-15-7 88%
Reaction Conditions:
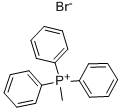
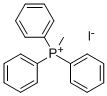
Stage #1: Methyltriphenylphosphonium bromidewith n-butyllithium in tetrahydrofuran;hexane at 0 - 20; for 4 h;Inert atmosphere;Wittig Olefination;
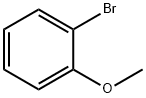
Stage #2: ortho-anisaldehyde in tetrahydrofuran;hexane; for 1 h;Inert atmosphere;Wittig Olefination;
Steps:
4.1.10 General procedure for preparation of compounds 20a-20g
General procedure: To a solution of Methyltriphenylphosphonium bromide (40.1mmol) in anhydrous THF (100mL) was added n-Butyllithium (2.4M in hexane, 17.9mL) at 0°C under Ar atmosphere. After stirred for 10min, the mixture was allowed to warm to room temperature and maintained with stirring for 4h until 19a-19g (3.6M in THF, 10mL) was added dropwise. Then the reaction solution was continuously stirred for 1h and Saturated solution of NH4Cl (10mL) was added slowly. Subsequently, the mixture was poured into water and the aqueous phase was extracted with DCM (100mL×3).The combined organic layers were washed with water and brine,dried over anhydrous Na2SO4. The dried solution was filtered and the filtrate was concentrated in vacuo. The crude product was purified by flash chromatography on a silica gel column to afford 20a-20g as colorless oil.
References:
Shi, Xiang;Du, Ting ting;Zhang, Zhihui;Liu, Xiaoyu;Yang, Ying;Xue, Nina;Jiao, Xiaozhen;Chen, Xiaoguang;Xie, Ping [Bioorganic Chemistry,2022,vol. 127,art. no. 106015]
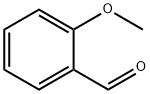
135-02-4
384 suppliers
$10.00/10g

612-15-7
72 suppliers
$59.39/1g