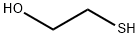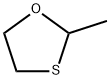
2-METHYL-1,3-OXATHIOLANE synthesis
- Product Name:2-METHYL-1,3-OXATHIOLANE
- CAS Number:17642-74-9
- Molecular formula:C4H8OS
- Molecular Weight:104.17
Yield:-
Reaction Conditions:
with toluene-4-sulfonic acid in dichloromethane;
Steps:
I Reaction: STR66 Procedure:
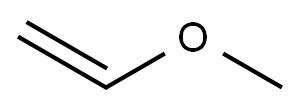
Reaction: STR66 Procedure: Into a 500 ml round-bottom flask is placed 250 ml dichloromethane. To the dichloromethane is added 39 grams of 2-mercapto ethanol. 24.2 grams of acetaldehyde is then added followed by 0.5 grams of p-toluene sulfonic acid. After boiling chips are added to the flask, a Dean-Starke distilling receiver is placed on the flask, and a reflux condenser is placed on the receiver. The flask is then heated slowly until reflux commences, and then the temperature is increased for a more vigorous reflux. The refluxing takes place over a period of 21/2 hours (until water of reaction ceases to be collected). The reaction mass is then cooled to room temperature and neutralized to a pH of about 8 with diethyl amine. The reaction mass is then filtered through fluted filter paper. The dichloromethane is removed from the reaction mass by means of rotary evaporation. 52.7 grams of crude product is then recovered. The resulting crude product is distilled by means of vacuum distillation, yielding the following fractions: The resulting material has a sulfury, metallic tomato aroma with a beet-like nuance and a sulfury, garlic, metallic flavor with a celery-like nuance. NMR, IR and Mass Spectral Analyses confirm that the struture of the resulting product is:
References:
US4042601,1977,A