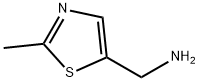
(2-METHYL-1,3-THIAZOL-5-YL)METHYLAMINE synthesis
- Product Name:(2-METHYL-1,3-THIAZOL-5-YL)METHYLAMINE
- CAS Number:63139-97-9
- Molecular formula:C5H8N2S
- Molecular Weight:128.2
![2-[(2-Methyl-5-thiazolyl)methyl]-1H-isoindole-1,3(2H)-dione](/CAS/20180808/GIF/838892-96-9.gif)
838892-96-9
2 suppliers
inquiry

63139-97-9
33 suppliers
inquiry
Yield: 61%
Reaction Conditions:
Stage #1:2-(2-methylthiazol-5-ylmethyl)isoindole-1,3-dione with hydrogenchloride;dibenzoyl peroxide in water for 7.5 h;Heating / reflux;
Stage #2: with sodium hydroxide in water
Steps:
15.1.C C. C- (2-METHYLTHIAZOL-5-YL) METHVLAMINE
Suspend 2- (2-METHYLTHIAZOL-5-YLMETHYL) ISOINDOLE-1, 3-dione (210 MG, D. 814 mmol) in 6N HCl (8 mL) and heat at reflux for 2.5 hours. Cool to room temperature and stir for an additional 5 hours. Basify the mixture with 2 N sodium hydroxide, extract with methylene chloride (3 x 50 mL), dry (sodium sulfate), filter, and concentrate to afford 70 mg (61% yield) of the title compound as a brown OIL. H NMR (CDC13) 6 7.34 (s, 1H), 4.11 (s, 2H), 2.45 (S, 3H), 1.70 (br s, 2H).
References:
ELI LILLY AND COMPANY WO2005/9941, 2005, A1 Location in patent:Page 31-32
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

63139-97-9
33 suppliers
inquiry
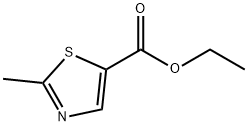
79836-78-5
131 suppliers
$13.43/250mgs:

63139-97-9
33 suppliers
inquiry
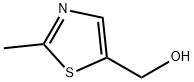
56012-38-5
59 suppliers
$45.00/5mg

63139-97-9
33 suppliers
inquiry
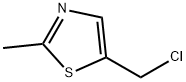
63140-11-4
33 suppliers
$65.00/100mg

63139-97-9
33 suppliers
inquiry