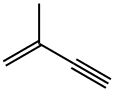
2-METHYL-1-BUTEN-3-YNE synthesis
- Product Name:2-METHYL-1-BUTEN-3-YNE
- CAS Number:78-80-8
- Molecular formula:C5H6
- Molecular Weight:66.1

335-03-5
0 suppliers
inquiry
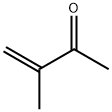
814-78-8
60 suppliers
$15.00/1g

338-68-1
0 suppliers
inquiry

78-80-8
61 suppliers
$45.32/5 G
Yield:338-68-1 33% ,78-80-8 10% ,814-78-8 7%
Reaction Conditions:
with aluminum oxide at 200; for 0.75 h;Inert atmosphere;Gas phase;Flow reactor;Pyrolysis;
Steps:
General procedure: Pyrolysis of gem-difluorocyclopropanes 1a-d in reactor B (in the presence of Al2O3). A quartz flow-tube reactor containing Al2O3 (10 g) was heated over 1 h at 450-550 °C in the flow of N2 (300 mL min-1) for the activation (removal of water) of Al2O3, then the reactor was cooled in the flow of N2 to 185 °C.Then, cis-3,3-difluoro-1,2-dimethylcyclopropane (1a) (3.00 g, 28.30 mmol) was passed at a constant rate through the reactor at 185 °C in the flow of N2 (70 mL min-1) over 45 min, using a syringe. The pyrolysis products coming out of the reactor were collected in a trap cooled with a mixture of CO2-propan-2-ol. The pyrolysate (1.97 g) was sequentially washed with 10% aqueous Na2CO3 and water, dried with Na2SO4, and distilled to obtain a mixture of the products (1.68 g, b.p. 45- 52 °C) containing according to the 1H and 19F NMR spectra (calculated using fluorobenzene as an internal standard) the starting cyclopropane 1a (0.27 mmol), (Z)-3-fluoropenta-1,3-diene (Z2a) (15.62 mmol), (E)-3-fluoropenta-1,3-diene (E2a) (1.95 mmol), and pent-1-en-3-yne 3a (1.53 mmol). The yields (in the mixture) were 55% (Z-2a), 8% (E-2a), 5% (3a).
References:
Volchkov;Lipkind;Novikov;Nefedov [Russian Chemical Bulletin,2015,vol. 64,# 3,p. 658 - 663][Izv. Akad. Nauk, Ser. Khim.,2015,# 3,p. 658 - 663,6]
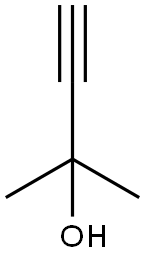
115-19-5
257 suppliers
$18.00/25mL

78-80-8
61 suppliers
$45.32/5 G

115-19-5
257 suppliers
$18.00/25mL

67-64-1
6 suppliers
$17.30/10ml

74-86-2
70 suppliers
inquiry

78-80-8
61 suppliers
$45.32/5 G
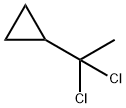
40459-85-6
6 suppliers
inquiry
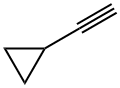
6746-94-7
323 suppliers
$13.00/1g
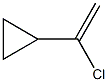
24154-06-1
4 suppliers
inquiry

78-80-8
61 suppliers
$45.32/5 G
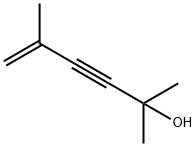
2696-26-6
4 suppliers
inquiry

78-80-8
61 suppliers
$45.32/5 G