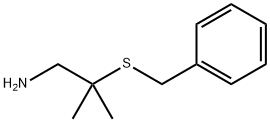
2-Methyl-2-(benzylthio)propylaMine synthesis
- Product Name:2-Methyl-2-(benzylthio)propylaMine
- CAS Number:59681-09-3
- Molecular formula:C11H17NS
- Molecular Weight:195.32
![Benzene, [[(1,1-dimethyl-2-nitroethyl)thio]methyl]-](/CAS/20210111/GIF/105780-12-9.gif)
105780-12-9
0 suppliers
inquiry

59681-09-3
6 suppliers
inquiry
Yield:59681-09-3 70%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether; for 1.71667 h;Cooling;Reflux;
Steps:
Synthesis of 2,2-dimethyl-2-benzylthioethylamine (2g)
Access to 2,2-dimethyl-2-benzylthioethylamine 2g wasgained following the method reported by Carroll et al.(1963). In the first step, a mixture of acetone (3.67 mL,0.05 mol), benzylmercaptan (5.90 mL, 0.05 mol), nitromethane(2.71 mL, 0.05 mol) and benzene (18.75 mL) wereheated at 100 °C inside a flask fitted with a Dean Starkapparatus filled with benzene. After 22 h, the crude mixturestill in the benzene solvent was left to cool to roomtemperature, transferred into a separatory funnel where itwas washed with 2.0 M HCl (20 mL × 2) and then withH2O(20 mL × 2). It was then dried with anhydrous MgSO4, concentrated in vacuo and purified by flash chromatographyusing n-hexane as eluent to yield 1-(benzylthio)-1,1-dimethylnitroethane2f (2.60 g, 23%). In the second step, a dryether solution of 1-(benzylthio)-1,1-dimethylnitroethane2f (1 g, 4.4 mmol) was added to a cold ether solution ofLiAlH4(4 M, 3.25 mL) inside a two-necked flask. The mixturewas left to stir for another 3 min after which it wastransferred to a heating block and heated under reflux for100 min. The crude mixture was transferred to a 250 mLflask and a stir bar added. H2Oand subsequently potassiumsodium tartarate (25 mL, 20% w/w) was added. Themixture was left to stir until all solids dissolved. The crudeproduct was then extracted with ether (20 mL × 3) andpurified by flash chromatography (16 - 50% ethyl acetate:n-hexane, ethyl acetate and finally methanol) to furnish2-(benzylthio)-2,2-dimethylmethylamine 2g as a yellow oil.Yield: (0.60 g, 70%), (cm-1) 3376 (NH), 1H (300 MHzCDCl3)7.40-7.19 (m, 5H, ArH), 3.68 (s, 2H, PhCH2S), 2.61(s, 2H, CH2NH2), 1.44 (s, 2H, NH2), 1.28 (s, 6H, SC(CH3)2);13C (75 MHz, CDCl3)138.5, 129.0, 128.7 and 127.1(ArC), 51.8 (C(CH3)2CH2NH2), 48.9 (C(CH3)2CH2NH2),32.8 (PhCH2S), 26.6 (C(CH3)2CH2NO2), m/z (ESI): 196[M + H]+.
References:
Aderibigbe, Abiodun D.;Clark, Andrew J. [Chemical Papers,2021,vol. 75,# 1,p. 397 - 410]
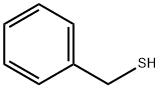
100-53-8
247 suppliers
$10.00/1g

59681-09-3
6 suppliers
inquiry

75-52-5
4 suppliers
$35.28/100g

100-53-8
247 suppliers
$10.00/1g

67-64-1
6 suppliers
$19.10/10ml

59681-09-3
6 suppliers
inquiry