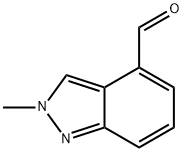
2-Methyl-2H-indazole-4-carboxaldehyde synthesis
- Product Name:2-Methyl-2H-indazole-4-carboxaldehyde
- CAS Number:1079992-61-2
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
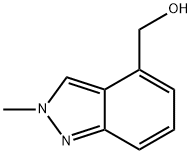
1079992-60-1
27 suppliers
$488.00/250mg

1079992-61-2
40 suppliers
$90.00/100mg
Yield:1079992-61-2 92%
Reaction Conditions:
Stage #1: (2-methyl-2H-indazol-4-yl)methanolwith oxalyl dichloride;dimethyl sulfoxide in dichloromethane; for 1 h;Inert atmosphere;
Stage #2: with triethylamine in dichloromethane at -78; for 1 h;
Steps:
4
Reference Example 4 2-methyl-2H-indazole-4-carbaldehyde Under nitrogen atmosphere, dimethyl sulfoxide (70.0 mL, 987 mmol) was added to a solution of oxalyl chloride (43.2 mL, 493 mmol) in dichloromethane (2.47 L) at -78°C, and the mixture was stirred for 2 hr. To the reaction mixture was added (2-methyl-2H-indazol-4-yl)methanol (40.0 g, 247 mmol), and the mixture was stirred for 1 hr. To the reaction mixture was added triethylamine (139 mL, 987 mmol) at -78°C, and the mixture was stirred for 1 hr and warmed to room temperature over 4 hr. To the reaction mixture was added aqueous ammonium chloride solution, and the mixture was extracted with dichloromethane. The extract was washed with saturated brine, and dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by recrystallization (dichloromethane/hexane) to give the title compound (36.3 g, yield 92%). 1H-NMR (CDCl3) δ: 4.29 (3H, s), 7.46 (1H, dd, J = 8.8, 6.8 Hz), 7.67 (1H, d, J = 6.8 Hz), 8.01 (1H, dd, J = 8.8, 0.8 Hz), 8.61 (1H, s), 10.1 (1H, s), MS (ESI+): 161 (M+H).
References:
EP2141150,2010,A1 Location in patent:Page/Page column 49