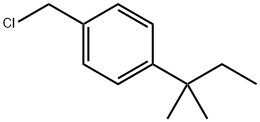
2-methyl-3-[4-(2-methylbutan-2-yl)phenyl]propanal synthesis
- Product Name:2-methyl-3-[4-(2-methylbutan-2-yl)phenyl]propanal
- CAS Number:67467-96-3
- Molecular formula:C15H22O
- Molecular Weight:218.33
Yield:67467-96-3 77%
Reaction Conditions:
with sodium hydrogencarbonate;palladium diacetate in N,N-dimethyl-formamide at 100; for 9 h;Product distribution / selectivity;Heck Reaction;
Steps:
1.b; 2
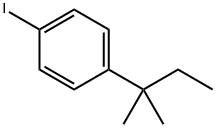
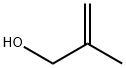
Through a mixture of (1,1-dimethyl-propyl)-4-iodo-benzene (137.07 g, 0.50 mol), palladium(II) acetat (1.122 g, 5 mmol) and sodium bicarbonate (50.40 g, 0.60 mol) in dry DMF (500 mL) was bubbled nitrogen for 10 min. Then 2-methyl-prop-2-en-1-ol (54.083 g, 0.75 mol) was added and nitrogen was bubbled for another ten minutes. The reaction mixture was heated under nitrogen for 9 h at 100°C. The reaction was cooled and was filtered through a thin layer of Celite. The Celite was then washed with DMF (300 mL). The reaction was then partitioned between H2O (2 L) and heptane/EtOAc (9/1) (2x1 L). The combined organic layers were washed with H2O (1 L) and were dried over MgSO4. The solvents were evaporated. 109.63 g of 3-[4-(1,1-dimethyl-propyl)-phenyl]-2-methyl-propionaldehyde were isolated as crude product. This intermediate was purified by distillation at 112°C under 0.06 mbar to afford 84 g of pure 3-[4-(1,1-dimethyl-propyl)-phenyl]-2-methyl-propionaldehyde (77% yield).. 1H NMR (400 MHz, CDCl3) δ: 0.69 (3H, t, J=7.45), 1.11 (3H, d, J=6.87), 1.29 (6H, s), 1.65 (2H, q, J=7.43), 2.60 (1HAB, dd, J= 8.18, 13.52 Hz), 2.69 (1H, sex, J=7.06), 3.08 (1H, dd, J = 5.87, 13.54 Hz), 7.12 (2H, d, J = 8.27), 7.27 (2H, d, J = 8.27), 9.75 (1H, s); 13C NMR (100 MHz, CDCl3) δ: 9.6 (CH3), 13.7 (CH3), 25.8 (CH3), 36.6 (CH2), 37.3 (CH2), 38.0 (C), 48.5 (CH), 126.4 (CHAr), 128.6 (CHAr), 136.0 (CAr), 148.0 (CAr), 205.1 (C=O).; Through a mixture of (1,1-dimethyl-propyl)-4-iodo-benzene (137.07 g, 050 mol), palladium(II) acetat (1.122 g, 5 mmol) and sodium bicarbonate (50.40 g, 0.60 mol) in dry DMF (500 mL) was bubbled nitrogen for 10 min. Then 2-methyl-prop-2-en-1-ol (54.083 g, 0.75 mol) was added and nitrogen bubbled for another ten minutes. The reaction mixture was heated under nitrogen for 9 h at 100°C. The reaction was cooled (0°C)and was filtered through a thin layer of Celite. The Celite was then washed with cold DMF (300 mL). To DMF solution was added EtOH (abs. 700 mL) followed by AcOH (30 mL) and cis-2,6-dimethylmorpholine (73.9 mL). After 15 min stirring at RT, the reaction was cooled to -5°C and NaBH4 (15.93 g, 0.42 mol) was added in portions over 1h 30 min so that temperature did not exceed 2-3°C. Stirring was continued at 0°C for 1 h and the reaction was quenched by drop-wise addition of NaOH solution in water at 0°C (30 g, 0.75 mol, /100 mL H2O) to pH=12. The reaction was then partitioned between H2O (2 L) and Hept/EtOAc (9/1) (2x1 L). The organic phases were combined, washed with the H2O (1L), and dried over MgSO4. After evaporation of the solvents, the residue (147.14 g) was dissolved in iPr2O (500 mL) and cooled to 0°C. To this solution was added the solution of HCl gas in EtOAc (150 ml, ~4M) drop-wise (30 min) and with stirring. White precipitate was formed during HCl addition and was filtered 30 min after completion of the addition. It was washed with iPr2O (300 mL) and was dried in vacuo at 40°C during 48 h providing 112.34 g of cis-4-{3-[4-(1,1-dimethylpropyl)-phenyl]-2-methyl-propyl}-2,6-dimethyl-morpholine hydrochloride as white powder. (64% yield) (Melting Point = 205.8°C) 1H NMR (400 MHz, CDCl3) δ: 0.63 (3H, t, J = 7.39 Hz), 1.12 (3H, d, J = 6.30 Hz), 1.13 (3H, d, J = 6.32 Hz), 1.24 (6H, s), 1.26 (3H, d, J = 6.65 Hz), 1.59 (2H, q, J = 7.49 Hz), 2.05 (1H, q, J = 10.77 Hz), 2.26 (1H, q, J = 10.80 Hz), 2.27-2.35 (1H, m), 2.56-2.66 (2H, m), 2.76-2.89 (2H, m), 3.22 (2H, t, J = 13.28 Hz), 4.36-4.44 (1H, m), 4.46-4.54 (1H, m), 7.06 (2H, d, J = 8.22 Hz), 7.23 (2H, d, J = 8.22 Hz), 12.55 (1H, b)
References:
EP1842848,2007,A1 Location in patent:Page/Page column 6; 7